Diagnosis of the Nutritional Status and Quality Assessment
of Oilseed Rape by X-Ray Spectroscopy
Schnug, E. and Haneklaus, S.
Institute of Plant Nutrition and Soil Science, Federal Agricultural Research Center Braunschweig-Voelkenrode, Bundesallee 50, D-38116 Braunschweig, Germany, ewald.schnug@fal.de
Abstract
Severe sulfur deficiency is at present, the most important nutrient disorder shown by northern European oilseed rape crops. The determination of the sulfur nutritional status by instrumental X-ray fluorescence spectroscopy provides a high level of accuracy, precision and performance in comparison with other methods. Performed on multi-channel spectrometers this technique can also provide data for other light elements such as calcium, chlorine, magnesium, phosphorus, potassium, silicon and sodium in the same run. Furthermore this procedure enables the indirect determination of the total glucosinolate content in seeds of Brassica species which is a standardized ISO method (ISO/CD 9167.2). Special sample preparation techniques for small sample sizes of vegetative tissues and seed material have been developed.
Keywords: Glucosinolates, X-ray fluorescence spectroscopy (XRF)
Introduction
The supply of oilseed rape with the major plant nutrients Ca, Mg, K, S and P as well as mineral elements such as Si, Cl and Na can be determined rapidly and precisely by X-ray spectroscopy. A special preparation technique has been developed which allows the analysis of vegetative tissue of oilseed rape in small sample sizes of 40 mg, compared to the usual 1 g. This is of particular interest when only small amounts of plant material are available. The glucosinolate content of Brassica seeds, being an important quality parameter, can be assessed efficiently by X-ray fluorescence spectroscopy (X-RF). The amount of seed required is 20 g, but special sample preparation techniques allow the analysis of much smaller amounts (e.g. individual seeds).
In this contribution the application of X-RF spectroscopy for the determination of light elements in vegetative plant tissue of oilseed rape and the indirect determination of glucosinolates in seeds of oilseed rape is described with special view to sample preparation and analysis of small sample sizes.
sample preparation techniques
Sample preparation of vegetative tissue of oilseed rape: normal sample sizes
Fresh, vegetative tissue of oilseed rape is dried in a ventilated oven at 85° C until constancy of weight. The dried plant samples are finely ground to a particle size <0.12 mm employing an ultra-centrifugal mill (RETSCH ZM1). Leaf tissue of oilseed rape can also be finely ground using a blender or coffee mill type grinder if crisp dry samples are to be milled. The finely ground material is mixed with HOECHST wax “C” in a ratio of 1:4 using a magnetic stirrer, in beakers, for 5 minutes. The stirrers employed are specially designed to produce a tumbling action which yields a homogenous mixture of sample material and wax. The amount of sample material required for the preparation of powdered parts should be in the range 0.9 – 1.1 g. The homogenate is then transferred into aluminum cups (diam.: 40 mm) and compacted by means of a hydraulic press at 1.2 t cm-2.
Sample preparation of vegetative tissue of oilseed rape: small sample sizes
Dried sample material is finely ground in a swing mill (RETSCH MM2) for 10 minutes at 80% capacity using zirconium or agate beakers in order to minimize losses during the grinding process. The fine ground material is mixed with HOECHST wax “microfine” in a ratio of between 1:40 and 1:80 on a magnetic stirrer, in beakers, for 5 minutes. The smallest sample size for analysis is 40 mg. A 1.25 g sample of the mixture is then compacted with a hydraulic press at 1.2 t cm-2 to a powdered part (diam.: 20 mm).
Sample preparation of oilseed rape seeds: normal sample sizes
The moisture content of oilseed rape seed samples should not exceed 7-9% which corresponds with the term ‘air-dry’. Otherwise 40 g (60 ml) of the seeds should be spread as a thin layer in a ventilated oven at 85° C for 75 minutes or in a microwave oven for 2 minutes at 600 W (Schnug and Haneklaus, 1990a). Samples need to cool down to room temperature before further processing. Spoon about 20 g (30 ml) of air-dry seed into a coffee mill type grinder and grind for 30 seconds. Scrape of any meal adhering to the wall of the blender by means of a spatula and grind the sample again for not more than 1 second in order to ensure homogenization. Fill to the brim either an aluminum cup (diam.: 40 mm) or a liquid cuvette (diam.: 40 mm) covered with mylar film (6 µm gauge) with the homogenized meal. Samples in aluminum cups are compacted by means of a hydraulic press at 1.0 t cm-2. Those in liquid cuvettes are compressed by a hand press under a pressure that reduces the volume of the meal to 35% (± 10%) of the original volume. A detailed description of sample preparation and possible errors are given by Anon (1991) and Schnug and Haneklaus (1990b).
Sample preparation of oilseed rape seeds: small sample sizes
Blenders are not suitable for sample sizes smaller than 15 g, but peppermills have been identified as suitable grinders for small sample sizes (ł 250 mg) of oilseed rape seeds. Details of this particular preparation technique are given by Schnug et al. (1993). After grinding, the seed material needs to be homogenized using a pestle and a small porcelain mortar. The homogenized material is evenly spread over the film covered bottom of a liquid cuvette (diam.: 20 mm) and gently compressed by means of a hand press. If sample sizes are less than 1 g, it is recommended that a lid of pure wax is prepared and placed on top of the liquid cuvette in order to prevent crackling of the thin meal layer during measurement.
Sample preparation of oilseed rape seeds: single seeds
Special seed holders need to be prepared for the embedding and analysis of single Brassica seeds. 5 g of HOECHST wax “C” are filled into an aluminum cup (diam.: 40 mm) and compressed by means of a hydraulic press at 1.2 t cm-2. A hemispherical hole is made in the center of the wax tablet using a metal ball which has been heated in the flame of a Bunsen burner. The hole size should be slightly broader than the diameter of a single seed. A S free glue is prepared (Schnug et al., 1993) by soaking 2 g of glucose in acetic acid for 1 hour in a porcelain crucible until the mixture is transparent. This mixture is then gently boiled. The tip of a pipette is heated in the flame of a Bunsen burner and a drop of the hot liquid is transferred to the hemispherical hole in the middle of the wax tablet. The seed is immediately placed in the center of the glue bed using tweezers. After hardening of the glue, the fixed seed is cut by means of a razor blade so that the surface of the intersection is parallel with the surface of the wax tablet. The diameter of the cut seed surface should be measured using precision sliding calipers.
Sample preparation of extracted oilseed rape meal
Extracted rapeseed meal samples are preheated in a microwave oven (600 Watt) for 60 sec and, either ground in an ultra-centrifugal mill or in a coffee mill type grinder. The meal can be directly compressed in aluminum parts or liquid cuvettes (see above).
X-RF SETTINGS
Fundamental technical requirements of X-RF for the analysis of plant material are given by Norrish and Chappell (1967). The spectrometer settings and measuring conditions for light elements by X-RF are summarized in table 1.
Table 1: Measuring conditions and practical detection limits of light elements by X-RF analysis.
|
Spectrometer type: Philips PW1400 X-ray tube: Rh Detector: Flow counter (Ar/CH4) Collimators: fine (0.15mm); coarse (0.55mm) |
||||||||||
Element |
Line |
Angle (2-theta) |
+Offset |
-Offset |
kV |
mA |
Collimator |
X-tal |
Conc. range (mg/g) |
LLD1 (µg/g) |
|
Mg |
Ka |
23.600 |
1.30 |
1.30 |
50 |
55 |
fine |
PX1 |
0.4 – 12.0 |
68.9 |
Na |
Ka |
28.530 |
1.30 |
1.56 |
50 |
55 |
coarse |
PX1 |
0.04 – 7.6 |
32.52 |
|
Cl |
Ka |
65.505 |
0.64 |
0.84 |
60 |
30 |
fine |
PE |
0.3 – 8.0 |
54.9 |
|
S |
Ka |
75.835 |
1.22 |
1.06 |
60 |
30 |
coarse |
PE |
0.5 – 20.0 |
20.8 |
|
P |
Ka |
89.535 |
0.66 |
1.22 |
60 |
30 |
fine |
PE |
0.2 – 11.0 |
37.7 |
|
Si |
Ka |
109.290 |
0.70 |
1.00 |
60 |
45 |
coarse |
PE |
0.5 – 19.0 |
26.3 |
|
Ca |
Ka |
113.215 |
1.92 |
1.56 |
60 |
30 |
fine |
LiF200 |
0.1 – 50.0 |
21.3 |
|
K |
Ka |
136.815 |
2.62 |
2.02 |
60 |
30 |
fine |
LiF200 |
1.0 – 27.0 |
45.1 |
|
note: 1LLD= Lower Limit of Detection with measuring time =20 sec; 2measuring time =200 sec |
||||||||||
With the exception of sodium measuring times of 20 seconds proved to be sufficient for normal sample sizes. The measuring time of small sample sizes needs to be extended to 40 to 400 seconds in dependence on the nutrient concentrations of the sample tissue. Sodium determination in these samples may not be possible due to the high dilution factor.
Calibration of X-RF for the determination of light elements in vegetative leaf tissue
Corrections for matrix effects and inter-elemental excitation are not necessary due to a sufficient dilution of the sample material by the wax. Standard reference materials (SRM’s; see Figure 1 and 2) of different sources are prepared using the methods outlined above. Calibration curves are shown for Si and Cl in Figure 1.
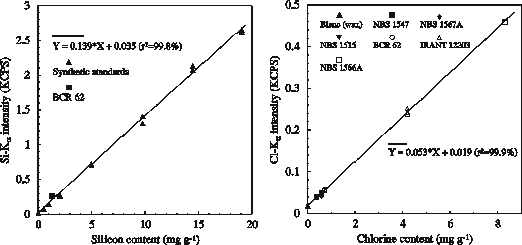
Figure 1. Relationship between certified silicon and chlorine contents in standard reference materials and Si-Ka and Cl-Ka intensities determined by X-RF.
Instrument stability and production quality of powdered parts were tested on both sample sizes by means of either a threefold sample preparation or a ten-fold measurement of one sample (Table 2).
Table 2. Influence of sample preparation and measuring repetitions on the concentration of light elements in celery leaves determined by X-RF on two sample sizes.
|
Content |
Ca |
K |
Mg |
Na |
P |
S |
Cl |
Si |
|
---------------------------------------------------- mg g-1----------------------------------------------------- |
||||||||
|
24.51 |
30.25 |
2.12 |
3.83 |
4.24 |
13.15 |
5.01 |
0.746 |
|
|
Ratio |
Coefficient of variation (%) of three repetitions of sample preparation |
|||||||
|
1:41 |
0.66 |
1.02 |
3.90 |
4.10 |
4.14 |
2.55 |
3.87 |
1.63 |
|
1:402 |
2.34 |
1.83 |
2.00 |
1.94 |
6.13 |
1.73 |
7.64 |
2.53 |
|
1:403 |
0.88 |
1.44 |
1.55 |
0.75 |
3.75 |
1.25 |
2.67 |
1.59 |
|
|
Coefficient of variation (%) of ten repetitions of elemental determination |
|||||||
|
1:4 |
0.32 |
0.33 |
3.22 |
4.00 |
1.75 |
0.43 |
4.99 |
1.39 |
|
1:40 |
0.67 |
0.83 |
2.61 |
0.43 |
3.08 |
0.36 |
2.73 |
1.31 |
|
note: 11:4 (1.1 g plant material : 4.4 g wax); 21:40 (40 mg plant material: 1.6 g wax); 31:40 with one repetition being the mean value of three powdered parts from each homogenate |
||||||||
For small sample sizes it is recommended to prepare three individual powdered parts of each homogenate in order to compensate for possible non-homogeneity. Small sample variation can be lower than that shown by the powdered parts of normal sample sizes (Table 2).
Calibration of X-RF for the determination of sulfur and glucosinlates in seeds of oilseed rape
For the initial calibration of the X-RF spectrometer three measurement samples of each certified reference material (CRM 190, CRM 366 and CRM 367) are prepared using the methods outlined above. The radiation intensity of the S-Ka line of the reference material is determined (Figure 2) under the routine operating conditions specified for the spectrometer (see table 1). Separate measurements are made on the three prepared sub-samples of each CRM material. For routine glucosinolate analysis, preparation of secondary rapeseed reference materials and secondary synthetic reference materials are recommended (see Schnug et al., 1992).
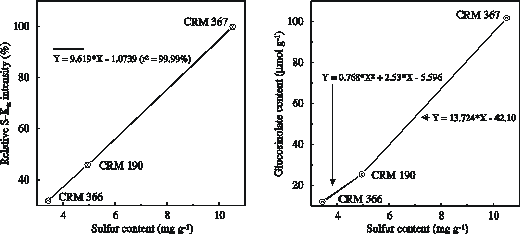
Figure 2. Relationship between the certified sulfur content of CRMs and the radiation intensity of the S-Ka line (left) and relationship between total sulfur content of CRMs and total glucosinolate content of CRMs (right).
The total glucosinolate content (µmol g-1) of unknown samples are calculated using a polynomial function up to a total S content in the seeds of 4.93 mg g-1 and a linear formula for higher concentrations (Figure 2). The sample preparation of CRMs for the measurement of small sample sizes is identical with that of normal sizes, with the only difference being that the ground seed material is filled and compressed in small liquid cuvettes like the unknown samples. The calibration is set as for normal sample sizes. During single seed analysis, the standard reference material and embedded seed samples are measured in a sample holder equipped with a titanium aperture of 26 mm width. Intensities of the S-Ka line are collected from the standard reference materials of normal sample sizes, an untreated wax tablet as blanc and the wax tablets with the embedded seed samples. The intensities of the embedded seed samples need to be transformed to values corresponding to the fluorescent area of the standard reference materials by means of the algorithm:
(counts seed sample – counts blanc) * (aperture radius)2 / (seed radius)2
The total sulfur content of the unknown sample is calculated by means of the regression of the measured count rates of single seeds and the certified sulfur concentrations in the CRMs. Calibration of X-FR spectroscopy for total sulfur determination in extracted rapeseed meal is carried out by the use of a SRM with certified sulfur content (e.g. NBS 1572) and standard addition of sulfur to extracted meal samples. All samples are diluted to 20% using wax. The algorithms for the calculation of the total glucosinolate content of the original seed from the total sulfur content of the processed meal are provided by Haneklaus and Schnug (1993).
Conclusion
X-RF spectroscopy is especially suited to the rapid and precise determination of light elements found in plant materials. Beside the nutritional status of oilseed rape, encompassing both essential macro-nutrients and mineral elements such as Cl, Na and Si, the quality of rapeseeds in terms of glucosinolates can also be assessed. Sophisticated sample preparation techniques enable the analysis of very small amounts of vegetative plant material and Seeds, whilst still maintaining their accuracy.
Acknowledgements
The authors thank Dr. R. Walker (SAC, Aberdeen) for improving the language of this paper.
References
Anon. 1991. ISO/CD (1991) 9167.2 Rapeseed - Determination of total glucosinolates - Part 2: X-Ray Fluorescence Spectrometry (XRF).
Haneklaus, S. and E. Schnug. 1993. Determination of total and destroyed glucosinolates in processed oilseed rape meal. Landbauforschung Voelkenrode 43: 73-76.
Norrish, K. and B. W. Chappell. 1967. X-ray fluorescence spectroscopy. in: J. Zusaman (ed) Physical methods in determinative mineralogy. Academic Press, London and New York.
Schnug, E. and S. Haneklaus. 1990a. Quantitative glucosinolate analysis in Brassica seeds by X-ray fluorescence spectroscopy. Phytochemical Analysis 1: 40-43.
Schnug, E. and S. Haneklaus. 1990b. A systematical study on factors influencing the determination of the total glucosinolate content in rapeseed by the X-RF method. Fat Sci. Technol. 92: 101-106.
Schnug, E., Boenke, A., Wagstaffe, P. J. and A. S. Lindsey. 1992. The characterisation of three rapeseed reference materials and their utilisation in the indirect determination of total glucosinolates in rapeseed by X-RF. Fat Sci. Technol. 94: 297-301.
Schnug, E., Murray, F. and S. Haneklaus. 1993. Preparation techniques of small sample sizes for sulphur and indirect total glucosinolate analysis in Brassica seeds by X-ray fluorescence spectroscopy. Fat Sci. Technol. 95: 334- 336.