ENHANCEMENT OF CHLOROPHYLL CLEARING IN MATURING
CANOLA SEED BY OVEREXPRESSING INVERTASE DURING SEED
MATURATION
Ian McGregor1, Shankar Das2, Brian Miki3, Wilf Keller2 and Ping Fu1
1Agriculture and Agri-Food Canada, 107 Science Place, Saskatoon, SK, Canada
S7N 0X2 (mcgregordi@em.agr.ca),
2National Research Council, Plant Biotechnology Institute, 110 Gymnasium Road, Saskatoon, SK,
Canada S7N 0W9,
3Eastern Cereal and Oilseed Research Centre, Agriculture Agri-Food Canada, K.W. Neatby Building,
Ottawa, ON, Canada K1A 0C6
ABSTRACT
A yeast-derived invertase, driven by the seed-specific napin storage protein promoter, was transformed
into Brassica napus L. cv Westar canola with overexpression targeted to the cytosol or the apoplast.
The intent was to precociously enhance chlorophyll clearing in maturing seed and thereby address the
"green seed problem". Homozygous plants were obtained with up to 12-fold increased expression over
Westar of soluble acid invertase in the cytosol. Overexpression of invertase targeted to the apoplast led
to increase in both soluble and insoluble acid invertase. Homozygous plants were obtained with up to
11-fold increased expression over Westar of total acid invertase. A developmental study with selected
lines expressing invertase targeted to the apoplast indicated that invertase expression did not deviate
over the filling phase of seed maturation. Relatively low levels of germination of R1 seed suggested
that appreciable overexpression of invertase, particularly when targeted to the apoplast, may interfere
with germination. Developmentally, neither the peak accumulation nor the timing or rate of chlorophyll
clearing was shown to be influenced by the achieved level of overexpression of apoplastic invertase.
KEYWORDS Brassica napus, apoplastic invertase, cytosolic invertase, Agrobacterium-mediated
transformation, seed chlorophyll content, germination
INTRODUCTION
The cotyledons of developing canola (Brassica napus L. and B. rapa L.) embryos are rich in chlorophyll
up to mid-maturation phase (500 to 800 g × g-1 fresh matter), then undergo a rapid programmed loss
that is usually completed well before the seed is mature (Johnson-Flanagan and Thiagarajah, 1990;
McGregor, 1991). When chlorophyll is retained in the mature canola seed as the result of an early frost
or other environmental factors (the "green seed problem") producers experience substantial economic
losses. Estimates of loss have ranged as high as 50 to 100 million in some years (Underwood, 1995).
As little as 3%distinctly green seed (>20 g × g-1 fresh matter; >20 ppm chlorophyll) reduces the
value of the crop. Chlorophyll extracts with the oil during processing (Yuen and Kelly, 1980; Appelqvist,
1989). Chlorophyll can inhibit the hydrogenation catalyst used for hardening in the manufacture of
margarine (Abraham and DeMan, 1986). Oils from seed with elevated chlorophyll content are less
stable, their oxidation resulting in rancidity (Dahléns, 1973). Chlorophyllides and pheophorbides,
phytol-deficient chlorophyll derivatives produced during processing, may contribute to photosensitive
dermatitis (Clare, 1955). Although technology exists for the removal of chlorophyll from the oil during
processing, removal adds to the cost of processing. Development of germplasm with improved ability to
clear chlorophyll before maturity is a permanent solution to reduce or eliminate green seed.
By varying the time of seeding, it has been shown that chlorophyll clearing in canola seed occurs at a
relative constant rate (McGregor, 1995). It has also been shown that the timing of chlorophyll clearing
may shift in relation to seed development (McGregor, 1995). Seeding early to ensure that the seed was
filling when temperatures were favourable, resulted in chlorophyll clearing occurring well in advance of
seed moisture loss. Seeding later so that the seed was filling under cooler temperatures, resulted in
chlorophyll clearing occurring along with the loss of seed moisture. Reduction in the temporal
separation between chlorophyll clearing and moisture loss would appear to contribute to elevated
residual chlorophyll content in mature seed. Chlorophyll becomes entrapped when moisture content of
the seed drops to the point that metabolic processes are curtailed and thus further breakdown of
chlorophyll can not occur.
Swathing studies have also shown that chlorophyll clearing occurs at a relatively constant rate once
initiated, and that swathing can advance the time of both chlorophyll clearing and moisture loss
(McGregor, 1995). If chlorophyll clearing was not underway at the time of swathing, swathing initiated
the process. The rate of clearing in the swathed crop was comparable to that which would have
subsequently occurred if the crop had been left standing. Thus, varying environmental conditions
during seed maturation pointed to the timing of the initiation of chlorophyll clearing as a potentially
important factor in determining the residual chlorophyll content of mature seed.
Organisms need to adjust their cellular metabolism and growth as a consequence of changes in nutrient
availability, developmental, and environmental signals. The capacity to monitor and respond to soluble
carbohydrate levels is an important adaptive mechanism, and hexokinase, the key enzyme that
catalyzes the first step in the glycolytic pathway (phosphorylation of hexose), has been implicated as a
glucose sensor in organisms as diverse as yeasts (Entain and Fröhlich, 1984; Rose et al., 1991) and
mammals (Efrat et al., 1994; Grupe et al., 1995). Recent results are consistent with the view that
hexokinase is also a bifunctional enzyme in plants. In addition to phosphorylating hexoses, it acts as a
sensor of soluble carbohydrate levels which, in turn, can activate or repress gene expression (Graham
et al., 1994; Jang and Sheen, 1994; Jang, et al., 1997). It is apparent that soluble carbohydrates affect
the expression of genes involved in many essential processes, such as glycolysis, glyoxylate
metabolism, nitrogen metabolism, defense mechanisms, cell cycle regulation, sucrose and starch
metabolism, and photosynthesis (Sheen, 1994; Koch, 1996). High carbohydrate levels repress the
expression of genes for carbohydrate production and induce genes for storage and utilization.
Carbohydrate depletion exerts opposite effects.
Using cellular systems, several groups have independently demonstrated that genes involved in
photosynthesis are repressed by glucose (Harter, et al., 1993; Krapp, et al., 1993; Jang and Sheen,
1994). Glucose transport alone is not sufficient to trigger repression. Glucose phosphorylation by
hexokinase is required. For example, the glucose analog 3-0-methylglucose, which is transported into
cells but not phosphorylated via hexokinase, does not trigger repression. Glycolytic intermediates
downstream of glucose, including the immediate phosphorylated product, glucose-6-phosphate, have
no effect on photosynthetic gene repression (Jang and Sheen, 1994). The glucose analog 2
-deoxyglucose, which is phosphorylated by hexokinase, but is not metabolized in the glycolytic pathway,
triggers a strong repression. Further, a hexokinase-specific inhibitor is able to reduce the glucose
repression of a maize photosynthesis-related gene (Jang and Sheen, 1994). Taken together, these
results indicate that phosphorylation of hexose by hexokinase is the site of soluble carbohydrate
sensing in plants (Graham et al., 1994; Jang and Sheen, 1994). However, although glucose
phosphorylation is important, cellular glucose content does not determine the strength of the signal.
Instead, metabolic flux through the hexokinase appears to be a critical factor. Regulatory function of a
bifunctional hexokinase is viewed as associated with a conformational change in the enzyme that
occurs transiently during the phosphorylation reaction.
In the present study, a yeast-derived invertase gene was introduced targeted to either the cytosol or
apoplast and under the control of the seed-specific napin storage protein promoter. The aim was to
increase the flux through the bifunctional hexokinase reaction by increasing the level of hexose
substrate (glucose and/or fructose). It was anticipated that increased flux would down-regulate genes
associated with synthesis of chloroplast differentiation, including chlorophyll a/b binding protein (Jang
and Sheen, 1994; Sheen, 1994), and perhaps chlorophyll itself. In any event, if sufficient chlorophyll a/b
binding sites were not available, newly synthesized chlorophyll would be degraded. By increasing
invertase at mid-maturity, chlorophyll clearing in the maturing seed would be precociously induced and,
because once induced the rate of clearing is more or less constant (McGregor, 1995), chlorophyll
clearing would be completed sooner.
EXPERIMENTAL METHOD
Transformation
Brassica napus L. cv Westar was transformed with with a recombinant DNA vector consisting of
pHS732, which is a pBIN19 derived vector (Bevan, 1984) that contains a 35S-35Spro-GUS-NPTII-nos
selection cassette (Kay et al., 1987) and the uid A (GUS) gene (Jefferson, 1987) of E. coli fused to the
neomycin phosphotransferase II gene. The trait gene, described in von Schaewen et al. (1990),
consisted of the coding region of the mature protein of the Saccharomyces cerevisiae suc2 gene
(Taussig and Carlson, 1983). Gene expresion was driven by the napin promoter from the napin gene
BngNAP1 (Baszczynski et al., 1990) isolated from B. napus cv Westar. Transformed material was
selected by GUS assay and for single copy insertions by Southern analysis. Invertase activity was
determined on developing seed tissue. Homozygous seed was selected by GUS assay of seed.
Brassica napus cv Westar was also transformed with a recombinant DNA vector consisting of pRD400,
which is a pBIN19 derived vector (Bevan, 1984) that contains the nos-wild type nptII-nos selection
cassette (Datla et al., 1992). The trait gene, described in von Schaewen et al. (1990), consisted of an
N-terminal sequence of the potato proteinase inhibitor II gene (Keil et al., 1986) fused in front of the
coding region of the mature protein of the Saccharomyces cerevisiae suc2 gene (Taussig and Carlson,
1983). Gene expresion was driven by the napin promoter from the napin gene BngNAP1 (Baszczynski
et al., 1990) isolated from B. napus cv Westar. The proteinase inhibitor II sequence targets the yeast
invertase to the cell wall and is cleaved during targeting. Transformed material was selected by
kanamycin assay and for single copy insertions by Southern analysis. As many single copy
independent transformations as possible were grown out, single plants selfed, and screened for
homozygous transformed and homozygous non-transformed by kanamycin assay of either maturing
seed or cotyledons of developing seedlings.
Sampling and analysis
Seed samples were collected during the filling phase of seed development to determine soluble
carbohydrate, starch, soluble and insoluble acid invertase, and chlorophyll content. Flowers were
tagged upon opening, brush pollinated and inflorescences bagged. Developing seed were collected
between 27 and 45 days after pollination (DPA), frozen in liquid nitrogen, and stored at -80%C.
Glucose, fructose and sucrose were determined enzymatically according to Stitt et al. (1989) and
expressed in mol × g fresh matter-1. Starch content was determined using the pellet remaining after
ethanol extraction according to Stitt et al. (1978) and expressed as g glucose equivalent seed part
-1. Soluble and insoluble invertase were assayed essentially according the method of (von Schaewen et
al., 1990). Chlorophyll content was determined using dimethylformamide (DMF) as the extraction
solvent (Morgan and Porath, 1980; Morgan, 1982) and expressed in parts per million (ppm).
RESULTS AND DISCUSSION
Transformation of the yeast invertase was initially targeted to the cytosol. Cytosolic expression of
invertase was originally chosen over apoplastic (or vacuolar expression) because studies with tobacco
had shown that plants were more sensitive to invertase expression in the cytosol, the cytosolic
invertase activity was highly expressed, and expressed earlier in leaf development (Sonnewald et al.,
1991). Subsequently, Frommer and Sonnewald (1995) noted that in developing potato tubers
expression of invertase in the cytosol led to reduced starch accumulation and yield while expression of
invertase in the apoplast led to improved tuber growth, with unaltered or only slightly reduced starch
content. Accordingly, the yeast invertase was also targeted to the apoplast.
In total 45 primary transformants (accessions) were produced expressing a single copy of the yeast
invertase gene targeted to the cytosol and restricted to cells in maturing B. napus cv Westar seeds with
the seed-specific storage protein napin promoter. Selfed (R1 seed) were obtained from 44 of these
primary transformants. Up to four R1 seeds of each independent transformation were grown out, seed
selfed, collected 30 days post anthesis (DPA), and the immature seed analyzed for soluble and
insoluble acid invertase activity. Homozygous lines were identified for 16 accessions. Soluble and
insoluble acid invertase activities for 30 DPA seed of the wild type were 0.042 and 0.094 mols min
-1 g fresh matter, respectively. For the homozygous plants, soluble and insoluble acid invertase
ranged up to 0.527 and 0.262 mols min g fresh matter, respectively. The highest soluble
acid invertase represented a 12.5 fold increase over Westar. Heterozygous plants showed less
variability in soluble acid invertase suggesting that differences would be easier to detect by screening
homozygous plants.
In total 40 primary transformants (accessions) were produced expressing a single copy of the yeast
invertase gene targeted to the apoplast and restricted to cells in maturing B. napus cv Westar seeds
with the seed-specific napin storage protein promoter. Selfed (R1 seed) were obtained from 35 of these
primary transformants.
From the earliest produced transformants both homozygous transformed and homozygous
nontransformed seed were identified for three lines, 1781, 1785 and 2077, and, based on the relatively
high invertase expression, these lines were selected for further study. Seed was grown out and 30 DPA
maturing seed collected for analysis of soluble and insoluble acid invertase activity, soluble sugars
(glucose, fructose and sucrose) and starch. Total acid invertase for individual plants ranged up to 1.548
mols min g fresh matter. It was noted that when insoluble acid invertase was elevated in
the transformants soluble acid invertase was also elevated indicating that not all of the yeast invertase
may have reached the apoplast and been bound to the cell wall. The highest total acid invertase
represented an 11.3-fold increase over the mean for Westar and a 10.8-fold increase over the mean
value for the nontransformed plants of the same line.
Carbohydrate analysis revealed increases in glucose and fructose and declines in sucrose for
homozygous transformed versus non-transformed plants. The increase in glucose content was
approximately 2-fold. Starch data showed no consistent pattern.
Seed of two homozygous lines, 1781 and 1785, were gorwn in a growth chamber at 18/15°C day/night
and 18/6 h light/dark regime, respectively. And sampled at 3 day intervals between 27and 48days
post anthesis (DPA). Soluble acid invertase activity was comparable for homozygous transformed and
nontransformed plants of both 1781 (Fig. 1) and 1785 (Fig. 2) over the three week filling period.
Insoluble acid invertase was higher in the homozygous transformed plants compared to the
homozygous nontransformed plants for both lines (Figs. 1, 2). For both lines, chlorophyll content of
homozygous transformed and nontransformed plants was comparable both in the peak chlorophyll
content accumulated and in the rate and timing of its decline (Fig. 3, 4). The data indicate that
overexpression of the yeast invertase or, at least, the level of overexpression achieved with these lines
was not sufficient to impact on chloroplast development and the chlorophyll clearing process.
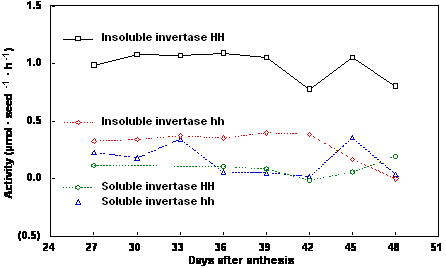
Figure 1. Soluble and insoluble invertase activity of maturing seeds from Brassica napus cv Westar
line 1781 homozygous non-transformed (hh) and homozygous transformed (HH) for yeast invertase
under the control of the seed-specific napin storage protein promoter and targeted to the apoplast.
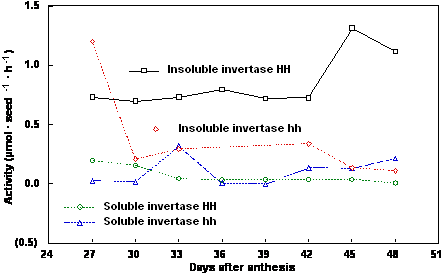
Figure 2. Soluble and insoluble invertase activity of maturing seeds from Brassica napus cv Westar
line 1785 homozygous non-transformed (hh) and homozygous transformed (HH) for yeast invertase
under the control of the seed-specific napin storage protein promoter and targeted to the apoplast.
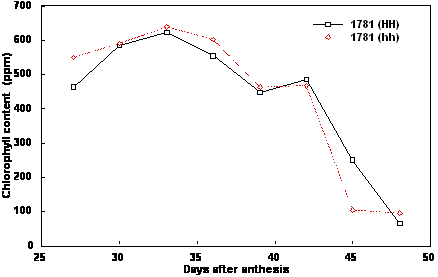
Figure 3. Chlorophyll content of maturing seeds from Brassica napus cv Westar line 1781 homozygous
non-transformed (hh) and homozygous transformed (HH) for yeast invertase under the control of the
seed-specific napin storage protein promoter and targeted to the apoplast.
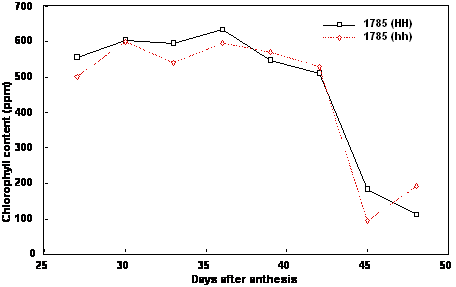
Figure 4. Chlorophyll content of maturing seeds from Brassica napus cv Westar line 1785 homozygous
non-transformed (hh) and homozygous transformed (HH) for yeast invertase under the control of the
seed-specific napin storage protein promoter and targeted to the apoplast.
Interestingly, insoluble acid invertase activity did not decline over the filling period (Figs. 1, 2). In
addition, it was observed that many cytosolic and apoplastic transformants germinated poorly. For
example, of 379 R1 seeds from 27 cytosolic transformants planted to select for homozygosity, 70 failed
to germinate, and of 518 R1 seeds from 30 apoplastic transformants planted to select for homozygosity,
290 failed to germinate. It is possible that high invertase expresssion, particularly in the apoplast,
impeded germination. Recently, Weber and coworkers (Weber et al., 1998) reported on attempts to
change the sugar status in developing seed of narbon bean (Vicia narbonensis) by overexpressing a
yeast-derived invertase gene under control of the LeguminB4 seed storage protein promoter. A signal
sequence targeted the invertase to the apoplast in maturing embryos. In the cotyledons, sucrose was
decreased whereas hexoses strongly accumulated, similar to the results for apoplastic expression in
Westar. Transgenic seeds were found to germinate so poorly that Weber and coworkers were
constrained to analyzing the segregating population of single seeds (R1). It was not possible to
generate homozygous transgenic lines of the stronger expressors.
Soluble carbohydrates affect the expression of genes involved in many processes (Sheen, 1994; Koch,
1996). In addition to the synthesis of chloroplast components, germination has been reported to be
influenced by the effect of soluble carbohydrates on gene expression (Zhou et al., 1998).
Seed of a glucose-insensitive mutant identified in Arabidopsis (gin1) was recently shown to germinate
faster (Zhou et al., 1998). Insensitivity to glucose repression of cotyledon and shoot development was
phenocopied by ethylene precursor treatment of wild-type plants or by constitutive ethylene
biosynthesis and constitutive ethylene signalling mutants, while an ethylene insensitive mutant
exhibited glucose hypersensitivity. GIN1 was postulated to balance the control of plant development in
response to metabolic and hormonal stimuli that act antagonistically. It was postulated that
phosphorylation of glucose via hexokinase would lead to the accumulation of GIN1 which, in turn,
would block ethylene promotion of germination (Zhou et al., 1998).
As with the overexpression of a yeast-derived invertase gene in developing seed of narbon bean
(Weber et al., 1998), in the present study, overepression of a yeast-derived invertase in Westar may
result in increased flux through a carbohydrate-sensing hexokinase leading to reduced germination.
Recently Trethewey and coworkers (Trethewey et al., 1998) introduced a bacterial glucokinase from
Zymomonas mobiles into an transgenic line of potato overexpressing a yeast-derived invertase. They
had previously noted that specific expression of a yeast invertase in the cytosol of tubers led to a
reduction in sucrose content, a reduction in starch, and an accumulation of glucose (Sonnewald et al.,
1997). Transgenic lines were obtained with up to threefold more glucokinase activity than in the parent
invertase line. There was a further dramatic reduction in starch content, down to 35% of wild-type
levels and no accumulation of glucose. Biochemical analysis of growing tuber tissue revealed large
increases in the metabolic intermediates of glycolysis, organic acids and amino acids, two- to threefold
increases in the maximum catalytic activities of key enzymes in the respiratory pathways, and three- to
fivefold increases in carbon dioxide production. These changes occurred in the lines expressing
invertase, and were accentuated following introduction of the second transgene, glucokinase.
Trethewey and coworkers concluded that the expression of invertase in the cytosol of potato tuber cells
leads to an increased flux through the glycolytic pathway at the expense of starch synthesis and that
heterologous overexpression of glucokinase enhances this change in partitioning.
In a further study Trethewey and coworkers (Trethewey et al., 1999) evaluated whether the localization
of sucrose cleavage had an impact on the glycolytic induction. Three additional transgenic potato lines
were used, one expressing ADP-glucose pyrophosphorylase in the antisense configuration, and two
double transgenic lines overexpressing a yeast-derived invertase targeted to either the cytosol or
apoplast specifically in tubers of the ADP-glucose pyrophosphorylase antisense line. It was found that
induction of the glycolitic enzymes only occured when the invertase was targeted to the cytosol, and
that the extent of this induction was comparable when invertase was overexpressed in the cytosol of in
the wild type (Sonnewald et al., 1997) or antisense ADP-glucose pyrophosphorylase backgrounds.
These results contrasted those of Herbers and coworkers (Herbers et al., 1996) who showed that
activation of plant defence mechanisms and repression of expression of photosynthetic genes occurred
when a yeast invertase was localized in the apoplast of tobacco leaves and not when it was targeted to
the cytosol. Trethewey and coworkers (Trethewey et al., 1999) conclude that the signal regulating
glycolysis is directly linked to cytosolic sucrose hydrolysis and hypothesised that signalling may be
associated with low cytosolic sucrose rather than flux through the hexokinase reaction per se.
Taken together, these studies would seem to indicate that if invertase is overexpressed in the cytosol,
storage capacity may be limited as partitioning is directed towards respiration (glycolysis). Repression
of photosynthetic gene expression is unlikely to occur because the cytosolic hexokinase in not
bifunctional. On the other hand, if invertase is overexpressed in the apoplast, repression of
photosynthetic genes may enhancing chlorophyll turnover but elevated invertase activity in the
developing seed must dissipate before seed maturity in order not to interfere with germination.
ACKNOWLEDGEMENTS
Technical assistance of D. Puttick, W. Friesen, G. Nowak, S. Campbell, D. Capcara and R. Wood is
greatfully appreciated. Financial assistance was received from the Canola Council of Canada and
Agriculture and Agri-Food Canada Matching Investment Initiative.
REFERENCES
Abraham, V. and De Man, J. M. 1986. Hydrogenation of canola oil as affected by chlorophyll. J. Amer.
Oil Chem. Soc. 63:1185-1188.
Appelqvist, L-Å. 1989. The chemical nature of vegetable oils. In: Rapeseed, Cultivation, Composition,
Processing and Utilization. L-Å. Appelqvist, and R. Ohlson (eds.). Elsevier Publishing Co, Amsterdam,
The Netherlands. pp. 123-173.
Baszczynski, C. L. and Fallis, L. 1990. Isolation and nucleotide sequence of a genomic clone
encoding a new Brassica napus napin gene. Plant Mol. Biol. 14: 633-635.
Bevan, M. 1984. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Research 12:
8711-8721.
Brown, C. S. and Huber, S. C. 1987. Photosynthesis, reserve mobilization and enzymes of sucrose
metabolism in soybean (Glycine max) cotyleodons. Physiol. Plantarum 70: 537-543.
Clare, N.T. 1955. Photosensitization in animals. Advances in Veterinary Sci. 2: 182-211.
Dahlén, J. A. H. 1973. Chlorophyll content monitoring in Swedish rapeseed and its significance in oil
quality. J. Amer. Oil Chem. Soc. 50: 312A-317A.
Datla, R., Hammerlindl, J., Panchuk, B., Pelcher, L. and Keller, W. 1993. Modified binary plant
transformation vectors with the wild-type gene encoding NPTII. Gene 211: 383-384.
Efrat, S., Tal, M. and Lodish, H. F. 1994. The pancreatic beta-cell glucose sensor. Trends Biochem.
Sci. 19: 535-538.
Entain, K-D. and Fröhlich, K-W. 1984. Saccharomyces cerevisiae mutants provide evidence of
hexokinase PII as a bifunctional enzyme with catalytic and regulatory dormains for triggering carbon
catabolite repression. J. Bacteriol. 158: 29-35.
Frommer, W. B. and Sonnewald, U. 1995. Molecular analysis of carbon partitioning in Solanaceous
species. J. Expt. Bot. 46: 587-607.
Graham. I. A., Denby, C. J. and Leaver, C. J. 1994. Carbon catabolite repression regulates glyoxylate
cycle gene expression in cucumber. Plant Cell 4: 761-772.
Grupe, A., Hultgren, B., Ryan, A., Ma, Y. H., Bauer, M. and Stewart, T. A. 1995. Transgenic
knockouts reveal a critical requirement for pancreatic beta cell glucokinase in maintaining glucose
homeostasis. Cell 83: 69-78.
Harter, K., Talke-Messerer, C., Barz, W. and Schafer, E. 1993. Light- and sucrose-dependent gene
expressionin photomixotrophic cell suspension cultures and protoplsts of rape (Brassica napus L.).
Plant Journal 4: 507-516.
Herbers, K., Meuwly, P., Frommer, W. B., Métraux, J.-P. and Sonnewald, U. 1996. Systemic
acquired resistance mediated by ectopic expression of invertase: possible hexose sensing in the
secretory pathway. Plant Cell 8: 793-803.
Jang, J. C. and Sheen, J. 1994. Sugar sensing in higher plants. Plant Cell 6: 1665-1679.
Jang, J. C., Leon, P., Zhou, L. and Sheen, J. 1997. Hexokinase as a sugar sensor in higher plants.
Plant Cell 9: 5-19.
Jefferson, R. A. 1987. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol.
Rep. 5: 387-405.
Johnson-Flanagan, A. M. and Thiagarajah, M. R. 1990. Degreening in canola embryos under
optimum conditions and following freezing. J. Plant Physiol. 136: 180-186.
Kay,R., Chan, A. and Daly, M. 1987. Duplication of CaMV 35S promoter creates a strong enhancer for
plan genes. Science 236: 1229-1302.
Keil, M., Sanchez-Serrano, J., Schell, J. and Willmitzer, L. 1986. Primary structure of a proteinase
inhibitor II gene from potato. Nucleic Acids Res. 14: 5641-5650.
Koch, K. E. 1996. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant
Mol. Biol. 47: 509-540.
Krapp, A., Hofmann, B., Schäfer, C. and Stitt, M. 1993. Regulation of the expression of rbcS and
other photosynthetic genes by carbohydrates: a mechanism for the sink regulation of photosynthesis.
Plant J. 3: 817-828.
McGregor, D. I. 1991. Influence of environment and genotype on rapeseed/canola seed chlorophyll
content. In: Proc. 8th Int. GCIRC Congress. July 9-11. Saskatoon, Canada. 6: 1743-1748.
McGregor, D. I. 1995. Chlorophyll clearing in developing canola seed. In: Proceedings of the GCIRC
9th International Rapeseed Congress. Cambridge, UK. July 4-7. pp. 506-508.
Morgan, R. and Porath, D. 1980. Chlorophyll determination in intact tissues using N,N
-dimethylformamide. Plant Physiology 65: 478-479.
Morgan, R. 1982. Formulae for determination of chlorophyllous pigments extracted with N,N
-dimethylformamide. Plant Physiology 69: 1376-1381.
Rose, M., Albig, W. and Entian, K-D. 1991. Glucose repression in Saccharomyces cerevisiae is
directly associated with hexose phosphorylation by hexokinase PI and PII. Eur. J. Biochem. 199: 511
-518.
Sheen, J. 1994. Feedback control of gene expression. Photosynth. Res. 39: 427-438.
Sonnewald, U., Brauer, M., Von Schaewen, A., Stitt, M. 1991. Transgenic tobacco plants expressing
yeast derived invertase in either the cytosol, the vacuole or the apoplast: a powerful tool to study
sucrose metabolism and sink-source interactions. Plant J. 1: 95-106.
Sonnewald, U. Hajirezaei, M. R., Kossmann, J., Heyer, A., Trethewey, R. N. and Willmitzer, L.
1997. Increased potato tuber size resulting from apoplastic expression of a yeast invertase. Nature
Biotechnology 15: 794-797.
Stitt, M., Wirtz, W. and Heldt, H. W. 1978. Pathway of starch breakdown in photosynthetic tissue of
Pisum sativum. Biochem. Biophys. Acta 544: 200-214.
Stitt, M., Lilley, R. M. C., Gerhard, R. and Heldt, H. W. 1989. Metabolite levels in specific cells and
subcellular compartments of plant leaves. Methods Enzymol. 174: 518-552.
Taussig, R. and Carlson, M. 1983. Nucleotide sequence of the yeast suc2 gene for invertase. Nucleic
Acids Res. 11: 943-1954.
Trethewey, R. N., Geigenberger, P., Riedel, K., Hajirezaei, M.-R., Sonnewald,U., Stitt, M.,
Reismeier, J. and Willmitzer, L. 1998. Combined expression of glucokinase and invertase in potato
tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 15:
109-118.
Trethewey, R. N., Geigenberger, P., Henning, A., Fleischer-Notter, H., Müller-Röber, B. and
Willmitzer, L. 1999. Induction of the activity of glycolytic enzymes correlates with enhanced hydrolysis
of sucrose in the cytosol of transgenic potato tubers. Plant Cell Environ. 22: 71-79.
Underwood, N. 1995. Stats Can pegs production at 6.586 million tonnes. In: Canola Digest. Canola
Council of Canada Publ. Winnipeg, Canada. October 1995. p. 4.
von Schaewen, A., Stitt, M., Schmidt, R., Sonnewald, U. and Willmitzer, L. 1990. Expression of a
yeast derived invertase in the cell wall of tobacco and Arabidopsis plants leads to inhibition of sucrose
export, accumulation of carbohydrates, and inhibition of photosynthesis, and strongly influences growth
and habitus of transgenic plants. EMBO J. 9: 3033-3044.
Weber, H., Heim, U., Golombek, S., Borisjuk, L., Manteuffel, R. and Wobus, U. 1998. Expression of
a yeast-derived invertase in developing cotyledons of Vicia naronensis alters the carbohydrate state
and affects storage functions. Plant J. 16: 163-172.
Yuen, W. and Kelly, P. 1980. The determination of chlorophyll in rapeseed oil. In: Analytical Chemistry
of Rapeseed and Its Products - A Symposium. J. K. Daun, D. I. McGregor and E. E. McGregor (eds.).
Canola Council of Canada Publ. pp. 139-143.
Zhou, L., Jang, J.-C., Jones, T. L. and Sheen, J. 1998. Glucose and ethylene signal transduction
crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 95:
10294-10299.