Sucrose content and freezing tolerance of Brassica napus canola (rapeseed) seedlings overexpressing an Escherichia coli inorganic pyrophosphatase
Ping Fu1, Jas Singh2, Wilf Keller3, and Ian McGregor1
1Agriculture and Agri-Food Canada, Saskatoon Research Centre, 107 Science Place, Saskatoon, SK, Canada S7N 0X2 (fup@em.agr.ca),
2Agriculture and Agri-Food Canada, Eastern Cereals and Oilseeds Research Centre, K.W. Neatby Building, Ottawa, ON, Canada K1A 0C6,
3National Research Council, Plant Biotechnology Institute, 110 Gymnasium Road, Saskatoon, SK, Canada S7N 0W9
ABSTRACT
Cold acclimation results in increased sucrose content which acts with other adaptations to confer freezing tolerance to plants. Inorganic pyrophosphate is a by-product of two reactions, pyrophosphate:fructose-6-phosphate-1-transferase and UDP-glucose pyrophosphorylase, leading to sucrose synthesis. Removal of pyrophosphate by overexpression of an inorganic pyrophosphatase (Ppase) was attempted to elevate sucrose levels of canola seedlings in response to low temperatures. A vector containing chimeric fusions of a cold inducible promoter (Bn115) with an E. coli ppa gene was constructed and introduced into the cytosol of B. napus L. cv. Westar by Agrobacterium-mediated transformation. After cold treatment for 7 days, ppase activity of the transformants was increased on average by 64 fold, whereas that of wild type remained unchanged. Cold acclimated transgenic seedlings also had higher levels of sucrose in cotyledons than did the wild type. There is a relatively strong correlation between ppase activity and sucrose content among the transformants (r=0.62). Freezing tolerance, determined as electrolyte leakage (%), was increased in the selected transformants after cold acclimation.
KEYWORD Brassica napus, inorganic pyrophosphatase, Agrobacterium-mediated transformation, cold acclimation, sucrose content, freezing tolerance
INTRODUCTION
Freezing tolerance in plants is an adaptive response to exposure to cool temperatures. For many plants, acclimation is associated with accumulation of soluble sugars in the cytosol, especially sucrose (Koster and Lynch, 1992). Soluble sugars are believed to protect both the cytoplasm and membrane structures during a subsequent freezing stress (Santarius, 1982). Formation of a supersaturated glass state liquid involving sugars is thought to prevent solutes from crystallizing as a result of a freeze induced dehydration of the cell (Koster, 1991). Membranes are believed to be protected by formation of hydrogen bonds between the hydroxyl groups of the sugar and polar residues of the membrane proteins or polar lipids (Crowe et al., 1992). Spring rapeseed varieties (B. napus and B. rapa) are sensitive to frost which can adversely affect stand establishment in Western Canada. Inability to increase sucrose content, and low activities of sucrose metabolic enzymes, in response to cold treatment have been identified as factors contributing to failure of spring rape to cold acclimate (Hurry et al., 1995). Introducing an inorganic pyrophosphatase (ppase) could potentially increase the flow of carbon towards sucrose by influencing two pyrophosphate producing reactions leading to sucrose synthesis, pyrophosphate: fructose-6-phosphate-1-transferase, and UDP-glucose pyrophosphorylase and by enhancing the export of triose phosphate from the chloroplast. To these ends, an inorganic pyrophosphatase (ppa) gene was overexpressed in canola in an attempt to enhance freezing tolerance.
Overexpression of E. coli inorganic pyrophosphatase in response to low temperatures
Spring rape (Brassica napus L. cv. Westar) was transformed with ppa gene from Escherichia coli (Sonnewald et al., 1992) under the control of the cold inducible Bn115 promoter (White et al., 1994). Sixteen F1 homozygous lines containing a single DNA insert and five lines containing multiple DNA inserts were identified. Initial screening identified two transgenic lines, 2120 and 2211 that contained either a single or multiple DNA inserts. Enzymatic activity of inorganic pyrophosphatase was assayed according to the method of Murphy and Riley (1962). After germination and growth at 18°C for 10 days, the transgenic seedlings were transferred to 5°C for up to 7 days and enzymatic activity of pyrophosphatase was analyzed. Ppase activity of both wild type and ppase transformants was not detected when grown at 18°C. Although increases in the ppase activity were evident in both wild type and the transgenic lines within 1 day of cold treatment (Fig. 1), ppase line 2211 which has multiple DNA inserts showed ppase activity (46.5 mmol PPi × min-1 × g dwt-1) about 6 fold higher than wild type controls (8.0 mmol PPi × min-1 × g dwt-1). While the increase in ppase activity of the transgenic line of 2240 was only marginal (9.1 mmol PPi × min-1 × g dwt-1). The higher levels of ppase activity in the transformants persisted throughout the 7 days of cold treatment with a the highest level occurring after 4 day of cold treatment. Analysis of sixteen ppase transgenic lines containing a single DNA insert and five lines containing multiple DNA inserts showed that the transformants containing multiple DNA inserts exhibited higher cold inducible ppase activity (average of 114 mmol PPi × min-1 × g dwt-1) compared to transformants containing a single DNA insert (average of 48.8 mmol PPi × min-1 × g dwt-1).
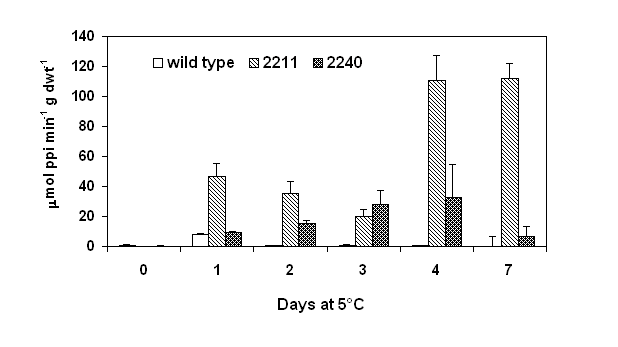 |
Figure 1. Pyrophosphatase activity of ppase transformants subjected to a cold treatment at 5°C
Changes in sucrose and starch content of the PPase transformants after cold treatment
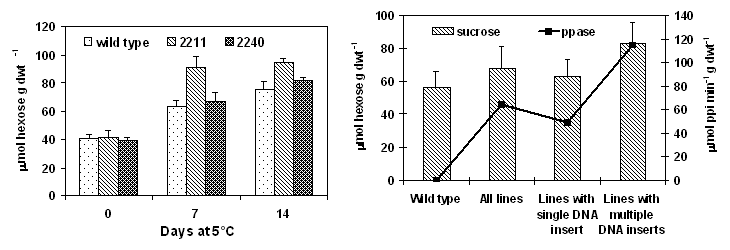
Glucose, fructose and sucrose of
transgenic seedlings overpressing the ppa gene were determined as
described by Stitt et al., (1989). After cold acclimating at 5°C for 7 days, the transgenic seedlings
showed higher levels of sucrose in cotyledons than did the wild type. After 7
days at 5°C, sucrose content was 91 mmol hexose equiv · g dwt-1 in
line 2211, 67 mmol hexose equiv · g
dwt-1 in ppase line 2240 and 63.4 mmol hexose equiv · g dwt-1 in wild type
(Fig. 2). Average sucrose content for transgenic seedlings with multiple DNA
inserts was higher than those with a single DNA insert (Fig.3). Cold acclimated
transgenic seedlings with a single DNA insert had an average sucrose content of
63 mmol hexose equiv · g dwt-1
compared to 82.7 mmol hexose equiv · g
dwt-1 for the seedlings containing multiple DNA. There was a strong
correlation between ppase activity and net sucrose increase among the
transformants (r=0.62). The higher sucrose content in the cotyledons of the
transgenic seedlings persisted for at least two weeks when seedlings were kept
at 5°C.
Figure 2. Increase in ppase activity in cotyledons Figure 3. Relationship between ppase activity and
of Brassica napus cv. Westar seedlings overexpressing sucrose content in cotyledons of Brassica napus cv.
ppa gene after subjected to a cold treatment at 5°C. Westar seedlings overexpressing the ppa gene.
The accumulation of starch in the ppase transgenic seedlings after being subjected to cold treatment was also examined. Ppase transgenic lines exhibited lower levels of starch content after cold treatment than the wild type. Transgenic lines containing single or multiple DNA inserts had an average starch content of 281 and 223 mmol hexose equiv · g dwt-1 respectively, compared to 367 mmol hexose equiv · g dwt-1 for the wild type. Moreover, analysis of total soluble carbohydrate content (glucose, fructose and sucrose) showed that the transformants containing multiple DNA inserts had higher levels of soluble carbohydrate than the transformants containing a single DNA insert or wild type. As ppase blocks sucrose transport from source to sink tissue (Geigenberger et al., 1996), no difference in leaf sucrose content between the transformants and wild type was expected or detected after cold treatment.
Freezing tolerance of ppase transformants
Detached cotyledons were frozen to various subzero temperature and freezing tolerance was determined as electrolyte leakage (%). Two transgenic lines, 2211 and 2120 expressing high activities of ppase and high levels of sucrose content were selected for the freeze test. The cotyledons of the transformants had significant lower electrolyte leakage than did the wild type after exposure to either gradually decreasing temperatures (Fig. 4) or to a prolonged period of exposure to -6°C (Fig. 5).
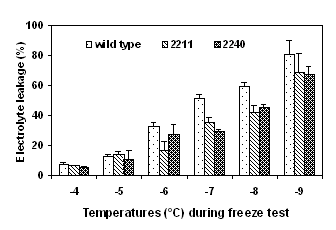
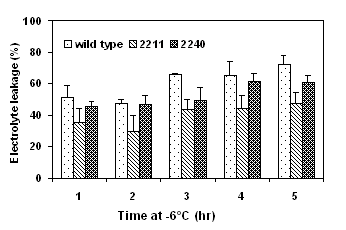
Figure 4. Freezing tolerance of ppase transformants Figure 5. Freezing tolerance of cotyledons of ppase
determined by a gradually decreasing temperature test. transformants determined by a time-course freeze test at -6°C.
CONCLUSIONS
Low temperature induction of pyrophosphatase overexpression in B. napus seedlings can be realized by a fusion of Bn115 cold-inducible promoter to a E. coli. inorganic ppa gene. Increased ppase activity led to an increase in sucrose content of cotyledons of ppase transformants. Elevation of sucrose levels enhanced freezing tolerance of the tissue tested. An increase in soluble carbohydrate content or a decease in starch content in high ppase expressers may increase osmolality of cells, thereby affecting the freezing tolerance of the tissue tested. As ppase blocks sucrose transport from source to sink tissue, sucrose content of sink tissue of the ppase transformants is not affected by overexpressing of ppa gene.
ACKNOWLEDGEMENTS
Thanks to S. Campbell, D. Capcara, T. Tackaberry, R. Wood and E. Ullathorne for technical assistance. Financial support from Performance Plant Ltd. is greatly appreciated.
REFERENCES
Crowe, J. H., Hoekstra, F. A. and Crowe, L. M. 1992. Anhydrobiosis. Annu. Rev. Physiol. 54: 579-599.
Geigenberger, P., Lerchl, J., Stitt, M. and Sonnewald, U. 1996. Phloem-specific expression of pyrophosphatase inhibits long-distance transport of carbohydrates and amino acids in tobacco plants.
Plant Cell and Environ. 19: 43-55.
Hurry, V. M., Strand, Å., Tobiæson, M., Gardeström, P. and Öquist, G. 1995. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism and carbohydrate content. Plant Physiol. 109: 697-706.
Koster, K. L. 1991. Glass formation and desiccation tolerance in seeds. Plant Physiol. 96: 302-304.
Koster, K. L. and Lynch, D. V. 1992. Solute accumulation and compartmentation during the cold acclimation of Puma rye. Plant Physiol. 98: 108-113.
Murphy, J. And Riley, J. P. 1962. A modified single solution method for determination of phosphate in natural water. Anal. Chim. Acta 27: 31-36.
Santarius, K. A. 1982. The mechanism of cryoprotection of biomembrane systems by carbohydrates.
In Li, D. A., Sakai, A. eds, Plant Cold Hardiness and Freezing Stress: Mechanisms and Crop Implications, Vol 2. Academic Press, New York, pp 475-486.
Sonnewald, U., Brauer, M., Von Schaewen, A. and Stitt, M. 1992. Transgenic tobacco plants expressing yeast derived invertase in either the cytosol, the vacuole or the apoplast: a powerful tool to study sucrose metabolism and sink-source interactions. Plant J. 1: 95-106.
Stitt, M., Lilley, R. M. C., Gerhard, R. and Heldt, H. W. 1989. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 174: 518-552.
White, T. C., Simmonds K., Donaldson P. and Singh, J. 1994. Regulation of Bn115, a low-temperature-responsive gene from winter Brassica napus. Plant Physiol. 106: 917-928.