Towards an understanding of genotypic variation in Brassica oleracea transformation efficiency
Judith A Irwin, Penny Sparrow and Philip J Dale
John Innes Centre, Colney Lane, Norwich, NR4 7UH, UK
ABSTRACT
Current transformation methods result in the gene of interest being introduced into selected genotypes amenable to transformation. The introduced gene is then transferred to the breeding line of choice by conventional cross-pollination. In this paper we describe the first part of a study designed to elucidate the genetic variation for a number of factors that affect transformation in Brassica oleracea.
As part of a programme to develop more repeatable and reliable Agrobacterium tumefaciens mediated transformation, more than 100 genotypes of six subspecies of B.oleracea were screened for adventitious shoot formation. We describe variation within and between subspecies of B.oleracea for adventitious shoot regeneration from hypocotyl segments and cotyledonary petioles of cabbage, cauliflower, broccoli, brussels sprouts, kale and kohl rabi. The frequency of shoot regeneration varied significantly between genotypes within subspecies from both explant types. Frequencies of shoot formation from cotyledon and hypocotyl explants from the same genotype were compared and the speed of shoot regeneration recorded. The benefits of using a standardized protocol to screen breeding lines for use in transformation programmes are discussed
KEYWORDS: shoot regeneration, tissue culture, hypocotyl, cotyledon
INTRODUCTION
Various factors are important for the efficient transformation of crop plants. Of these variables, adventitious shoot regeneration from explants amenable to transformation is one of the most important. Even when transformation has been achieved and is routine for a particular genotype of a crop species, it can still be difficult to transform any genotype of choice within that species. Numerous reports cite differences between genotypes within a species in the ability to respond in vitro and to regenerate shoots (e.g. Diertert et al 1982; Christey and Earle, 1991). Such reports indicate that the developmental processes reflected by in vitro response are genetically controlled.
The objective of this investigation is to examine the extent of the variation among subspecies of B.oleracea. Specifically, (1) how much genetic variation is there for adventitious shoot regeneration, (2) are there likely to be responsive genotypes within each of six important B.oleracea subspecies, and (3) is it worthwhile to test a range of breeding germplasm for lines with high morphogenic response.
MATERIALS AND METHODS.
Seeds from each of 102 genotypes were germinated on plates containing MS medium (Murashige and Skoog, 1962)), 3% sucrose and 0.8% phytagar. Hypocotyl and cotyledon explants were taken from 5-day-old seedlings. Cotyledons were excised from the cotyledonary node, taking great care to eliminate the apical meristem and leaving at least 2mm of petiole (after Moloney et al, 1989). Hypocotyls were cut into 6-8mm segments. Both hypocotyl and cotyledon explants were taken from each seedling. The explants were placed onto plates containing MS medium as before, with the addition of 2mg/l BAP. Explants were placed in a 23oC culture room with 16hr daylight of cool fluorescent light. Shoot regeneration was scored 16, 23 and 44 days after explants were taken (data from 16 and 23 days not shown). All experiments were replicated and randomsied within the culture room. Data were expressed as percentages and angular transformation used to make the assumption of normality more reasonable (Gilbert 1973). Analysis of variance was performed on the transformed data at 44 days, with genotypes nested within subspecies, to determine any significant differences within subspecies, and between subspecies and explant type.
RESULTS AND DISCUSSION
After 44 days, of the 102 genotypes tested, all regenerated some shoots from cotyledon explants and 100/102 regenerated shoots from hypocotyl segments. Within each of the six subspecies of B. oleracea, at least one genotype regenerated shoots at a frequency of 75% or better. After 16 days 91% of genotypes were showing some regeneration from cotyledons and 78 % from hypocotyl segments. After 23 days, 99% were regenerating from cotyledons and 93% from hypocotyls (data not shown). No increase in the percentage shoot regeneration was observed after 44 days. Table 1 shows mean percentage shoot regeneration from cotyledon and hypocotyl explants after 44 days.
Table 1: Mean percentage regeneration frequencies from hypocotyl and cotyledon explants of kale, cauliflower, cabbage, brussels sprouts, kohl rabi and broccoli. Standard error of the mean is given in parenthesis.
|
Brassica subspecies |
n |
cotyledon |
hypocotyl |
|
|
|
|
|
|
kale (B.oleracea acephala) |
10 |
66.39 (9.40) |
50.60 (9.79) |
|
cauliflower (B.oleracea botrytis) |
17 |
67.76 (4.60) |
57.92 (6.80) |
|
cabbage (B.oleracea capitata) |
22 |
80.89 (2.96) |
70.73 (3.94) |
|
brussels sprouts (B.oleracea italica) |
22 |
76.69 (5.11) |
37.47 (6.21) |
|
kohl rabi (B.oleracea gonglodes) |
11 |
70.48 (5.97) |
50.40 (8.83) |
|
broccoli (B.oleracea gemmifera) |
21 |
54.93 (5.23) |
60.96 (4.92) |
|
|
|
|
|
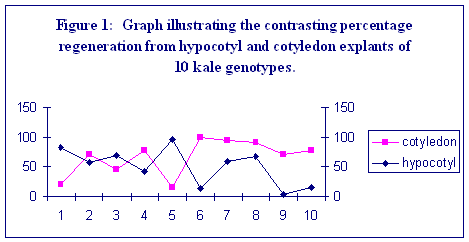 |
From both explant types, the highest overall frequency of regeneration was observed for cabbage genotypes. A high frequency of shoot regeneration from one explant type was not always correlated with a high frequency from the other. This is most clearly demonstrated within the six kale genotypes (Figure1). In other species it has been found that when genotypes are ranked for regeneration frequency from one explant, the ranking is often broadly the same when a different explant type is used (Dale and Hampson, 1995). However, within the kale genotypes used in this study, a negative correlation is displayed for regeneration frequency from cotyledonary petioles compared to hypocotyl explants (r = - 0.657, p=0.039). The basis of this difference is unclear. Regeneration from both explant types involves adventitious shoot formation, but hypocotyls display greater callus formation before shoots are formed. Statistical analysis of the data shows that there are significant differences between subspecies and, between genotypes within subspecies for both hypocotyl and cotyledon explants (p<0.001). The rate of regeneration was slower from hypocotyl explants compared to cotyledons, particularly for brussels sprout genotypes. There was more variation between genotypes for regeneration rate from hypocotyl explants than from cotyledonary petioles (illustrated in Table 2).
An analysis of the percentage components of variation accounted for by the three main effects (time, explant type and genotype), interactions between the main effects and the error associated with these measurements was carried out on a subset of 68 genotypes. For kale, 76% of the variation is accounted for by the interaction between explant type and genotype, indicating that within this subspecies, the performance of a genotype depends to a great extent on the explant type being cultured.
Table 2: Mean percentage shoot regeneration frequencies from cotyledon and hypocotyl explants of a subset of kale, broccoli and cabbage genotypes as examples of the variation found within subspecies. Letters (a,b,c,d) indicate significant differences between genotypes (Tukeys comparison). Genotypes with the same letter are not significantly different (p>5%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Line |
Cot. |
|
|
|
|
Hyp. |
|
|
|
|
|
Line |
Cot. |
|
|
|
Hyp. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
K1 |
26.6 |
a |
|
|
|
77.9 |
|
|
|
d |
e |
CB1 |
57.8 |
a |
|
|
56.4 |
a |
b |
c |
|
|
|
|
K2 |
68.5 |
|
b |
c |
|
58.2 |
|
b |
c |
|
|
CB2 |
64.2 |
a |
b |
c |
67.6 |
|
b |
c |
d |
e |
|
|
K3 |
50.6 |
a |
b |
|
|
67.4 |
|
|
c |
d |
|
CB3 |
76.0 |
a |
b |
c |
75.3 |
|
|
c |
d |
e |
f |
|
K4 |
72.9 |
|
|
c |
|
48.3 |
|
b |
|
|
|
CB4 |
60.3 |
a |
b |
c |
81.6 |
|
|
|
d |
e |
f |
|
K5 |
25.5 |
a |
|
|
|
92.4 |
|
|
|
|
e |
CB5 |
77.3 |
a |
b |
c |
80.9 |
|
|
|
d |
e |
f |
|
K6 |
100 |
|
b |
c |
|
26.2 |
a |
|
|
|
|
CB6 |
79.4 |
a |
b |
c |
49.3 |
a |
b |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CB7 |
54.4 |
a |
|
|
78.6 |
|
|
|
d |
e |
f |
|
BR1 |
38.7 |
a |
b |
|
|
73.0 |
|
b |
c |
|
|
CB8 |
87.4 |
a |
b |
c |
60.6 |
|
b |
c |
d |
|
|
|
BR2 |
48.8 |
a |
b |
c |
|
44.7 |
a |
b |
c |
|
|
CB9 |
84.6 |
a |
b |
c |
73.1 |
|
|
c |
d |
e |
|
|
BR3 |
70.6 |
|
|
c |
d |
61.4 |
a |
b |
c |
|
|
CB10 |
86.6 |
a |
b |
c |
81.2 |
|
|
|
d |
e |
f |
|
BR4 |
84.9 |
|
|
|
d |
81.3 |
|
|
c |
|
|
CB11 |
95.2 |
|
|
c |
71.6 |
|
|
c |
d |
e |
|
|
BR5 |
88.5 |
|
|
|
d |
69.3 |
|
b |
c |
|
|
CB12 |
94.4 |
|
b |
c |
86.2 |
|
|
|
|
e |
f |
|
BR6 |
63.8 |
|
b |
c |
d |
73.4 |
|
b |
c |
|
|
CB13 |
85.0 |
a |
b |
c |
84.6 |
|
|
|
|
e |
f |
|
BR7 |
79.3 |
|
|
c |
d |
34.7 |
a |
b |
|
|
|
CB14 |
74.1 |
a |
b |
c |
68.9 |
|
b |
c |
d |
e |
|
|
BR8 |
32.6 |
a |
|
|
|
27.3 |
a |
|
|
|
|
CB15 |
97.1 |
|
|
c |
95.2 |
|
|
|
|
|
f |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In contrast for brussels sprouts, only 14% of the variation is accounted for by this interaction term, while time and explant type are responsible for the largest proportion of the variation. In broccoli the most important factor is genotype, while for cabbage, it is time, suggesting differences between genotypes within this subspecies in the speed of regeneration and overall regeneration frequency.
The difference in ability of genotypes to regenerate from different explants presumably reflects variation in the genetic potential to regenerate through different morphogenic routes. The greater variability in regeneration frequency observed from hypocotyl explants compared to cotyledonary petioles, particularly in cabbage and brussels sprout genotypes, presumably reflects the longer callus phase involved in regeneration from hypocotyl segments. In some subspecies, especially brussels sprouts, there is a difference between genotypes both in ability to regenerate under the culture conditions used and in the speed with which the different genotypes respond. Studies currently underway in the laboratory will determine whether there is any relationship between speed of regeneration of cotyledonary petioles and susceptibility to Agrobacterium mediated transformation.
Regeneration of shoots from cotyledons and hypocotyls has been reported in several Brassica species including B. oleracea (Dietert et al., 1982; Dale and Ball, 1991; Kumar et al., 1993). These reports have shown considerable variation in regeneration frequency between genotypes. However, in most cases, only one source tissue was used and the number of genotypes investigated was very limited. In this study, plants were regenerated from cotyledonary explants of all 68 genotypes of B.oleracea investigated, and from hypocotyl explants taken from 98% (67/68) of the genotypes under study. Regeneration frequencies were found to vary considerably between subspecies of B.oleracea, between genotypes within each subspecies and between explant types. Genotypic influence on in vitro morphogenesis in Brassica has been documented previously (Dietert et al., 1982; Dunwell, 1981; Fazekas et al., 1986; Khehra and Mathias, 1992). The importance of genotype in influencing the frequency of regeneration in B. oleracea is supported by this much larger study.
The ability to genetically modify plants combines components of plant tissue culture and Agrobacterium related factors such as virulence and T-DNA transfer and integration. However, the routine use of genetic modification in plant breeding for the development of new cultivars requires that the technology is applicable to a wide range of genotypes. Selection of breeding lines that are genetically predisposed to shoot regeneration is therefore important. The results reported here show that testing a range of material for lines with high morphogenetic response using a simple standardised culture medium provides a cost effective approach to selecting genotypes for use in GM plant breeding. In cases where a number of lines are potentially useful, this approach could prove much more efficient than attempting to maximise the number of genotypes used by making a myriad of small changes to the culture medium. However, we have found that from cotyledonary explants most genotypes possess a significant level of regeneration potential. Therefore in situations where transferring a gene to a single genotype is highly desirable, with a little more effort this goal should be achievable.
CONCLUSION
All subspecies of B.oleracea contain sufficient genetic variability for adventitious shoot formation that one or more lines within a subspecies could be used for transformation. This means that lengthy backcross programmes between different vegetable types should prove unnecessary and hence save valuable time in bringing new varieties to the market place.
The elucidation of the genetic basis of a number of variables important in the transformation process will provide valuable information on which genotypes would be best used by breeders for a GM approach. This study has shown that considerable variation exists within each of six subspecies of B.oleracea for potential regeneration of adventitious shoots. We have further shown that a single standardised culture medium can be used to obtain high levels of regeneration from a large range of genotypes. Further investigations currently underway in the laboratory will determine the number and location of genes responsible for shoot regeneration and other factors effecting transformation. One part of this study is described in paper 796: Mapping QTLs for transformation variables in Brassica oleracea.
Acknowledgments
We thank Horticultural Research International Genetic Resources unit, A.L.Tozers Ltd. and Elsoms Seeds for supplying the seed stocks used in this study, and the BBSRC for their support. This work was funded by the UK Ministry of Agriculture Fisheries and Food (Contract number HH1103SVF)
References
Christey MC, Earle ED (1991) Regeneration of Brassica oleracea from peduncle explants. Hortscience 26: 1069-1072
Dale PJ, Ball LF (1991) Plant regeneration from cotyledonary explants in a range of Brassica species and genotypes. Proceedings of the Eighth International Rapeseed Congress 4: 1122-1127
Dale PJ, Hampson KK (1995) An assessment of morphogenic and transformation efficiency in a range of varieties of potato (Solanum tuberosum L.) Euphytica 85: 101-108
Dietert MF, Barron SA, Yoder OC (1982) Effects of genotype on in vitro culture in the genus Brassica. Plant Sci Let 26: 233-240
Dunwell JM (1981) In vitro regeneration from excised leaf discs of three Brassica species. J Exp Bot 32: 789-799
Fazekas GA, Sedmach PA, Palmer MV (1986) Genetic and environmental effects on in vitro shoot regeneration from cotyledonary explants of Brassica juncea. Plant Cell Tiss Organ Cult 6: 177-180
Gilbert N (1973) Biometrical interpretation. Oxford University Press
Kellar WA, Armstrong KC (1977) Embryogenesis and plant regeneration in Brassica napus anther cultures. Can J Bot 55: 1383-1388
Khehra GS, Mathias RJ (1992) The interaction of genotype, explant and media on the regeneration of shoots from complex explants of Brassica napus L. J Exp Bot 43: 1413-1418
Kumar A, Kumar VA, Kumar J (1993) Rapid in vitro propagation of cauliflower. Plant Sci 90: 175-178
Moloney MM, Walker, JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8: 238- 242
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473-497