EPIDEMIOLOGY, FORECASTING
AND MANAGEMENT OF WINTER OILSEED RAPE DISEASES IN THE UK
B D L Fitt1,
P Gladders2, K G Sutherland3, J A Turner4, S J
Welham1
1IACR-Rothamsted,
Harpenden, Herts. AL5 2JQ,
2ADAS
Boxworth,
3SAC,
Bucksburn,
4Central
Science Laboratory, MAFF, Sand Hutton,
ABSTRACT
Survey results show that the most serious diseases on winter oilseed rape in the UK are light leaf spot (Pyrenopeziza brassicae) and stem canker (Leptosphaeria maculans), then stem rot (Sclerotinia sclerotiorum) and dark pod spot (Alternaria brassicae); severity of epidemics differs between seasons, between regions and between crops. Diseases cause losses of up to £80M each season, despite expenditure of up to £12M on fungicides to control them. Light leaf spot is a polycyclic disease initiated in autumn (GS 1,05), probably by wind-borne ascospores, and spread by splash-dispersed conidia. Stem canker is a monocyclic disease; in autumn wind-borne ascospores infect leaves to cause phoma leaf spots and the pathogen grows down petioles to stems, on which canker lesions are observed in spring (GS 6,4). Regional forecasts predict risk of severe light leaf spot epidemics and can be modified by specific factors (e.g. cultivar, sowing date) to estimate risks in individual crops. A protocol for confirming the presence of light leaf spot involves sampling crops, incubating plants and assessing the appearance of diagnostic white pustules of the pathogen. Field experiments have suggested that growers need to consider control of light leaf spot and stem canker in autumn and late winter and control of stem rot and dark pod spot in spring.
KEYWORDS: Disease surveys, fungicide timing, light leaf spot, sampling, stem canker, yield loss.
INTRODUCTION
Integrated
management of diseases on winter oilseed rape, the most important arable crop
in the
ESTIMATING LOSSES FROM
DISEASES
Retrospectively,
losses from diseases have been estimated by combining data from the ADAS/CSL
winter oilseed rape disease survey for
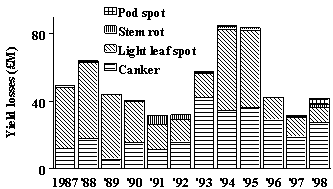
Fig. 1. Winter oilseed rape
disease losses in
An
equation for prediction of yield loss from light leaf spot incidence on leaves
at growth stage (GS) 3,3 (flower buds visible; Sylvester-Bradley &
Makepeace, 1985) has been developed (Su et
al., 1998). This relationship was based on results from experiments in
EPIDEMIOLOGY OF LIGHT LEAF SPOT AND STEM CANKER
Not
only are light leaf spot and stem canker the two most important diseases of
winter oilseed rape in the UK, but also they differ greatly in their
epidemiology (Table 1). Light leaf spot
is a polycyclic disease, which infects leaves, stems, flowers and pods, with
several pathogen life cycles occurring
between sowing in August/September and harvest the following July
(McCartney & Lacey, 1990). By
contrast, stem canker is a monocyclic disease, with one life cycle per season,
although there is an extended period from October to March over which stem
lesions may be initiated by infection of different leaves (Gladders & Musa,
1980; Hammond & Lewis, 1986). Light
leaf spot is caused by one pathogen, Pyrenopeziza
brassicae, although there is evidence of genetic variability within the
Table
1. Contrasting epidemiology of light
leaf spot (Pyrenopeziza brassicae)
and stem canker (Leptosphaeria maculans)
on winter oilseed rape in the UK
|
|
Light leaf spot |
Stem canker |
|
Epidemics |
Polycyclic |
Monocyclic |
|
Population |
One
type |
Two
types |
|
Primary
inoculum |
Ascospores? Conidia? |
Air-borne
ascospores |
|
Colonisation |
Non-systemic? |
Systemic (leaf ®stem) |
|
|
Long
latent period |
Long
incubation period |
|
Secondary
spread |
Splashed
conidia |
None? |
|
Damage |
Leaves,
Pods |
Stems |
|
Regional
importance (UK) |
North,
West & Midlands |
East,
South & Midlands |
The
primary inoculum for stem canker epidemics is air-borne L. maculans ascospores, produced in pseudothecia on stem debris
from previous crops (Gladders & Musa, 1980), which infect leaves to cause
phoma lesions in autumn (GS 1,05). However, the role of air-borne ascospores of
P. brassicae, produced in apothecia
on stem and pod debris, in initiating epidemics of light leaf spot is less
clear (McCartney & Lacey, 1990) and splash-dispersed conidia produced in
acervuli on volunteer oilseed rape seedlings and vegetable brassica crops may
also be involved. The optimum
temperature for infection of oilseed rape leaves by L. maculans ascospores is c.
20°C; at this temperature the maximum number of lesions is greater than at
lower (Fig. 2a) or higher temperatures (Biddulph et al., 1998). The optimum wetness duration at 20°C is c. 48 h; at shorter wetness durations
(Fig. 2b) fewer lesions are formed and at longer wetness durations no more
lesions are formed. By contrast, the
optimum temperature for infection by P.
brassicae conidia is c. 15°C
(Figueroa et al., 1995). From
infected leaves, L. maculans grows
systemically down leaf petioles (on which no symptoms are visible) to reach the
stems, on which lesions appear the following spring (GS 6,4; Hammond et al., 1985; Hammond & Lewis,
1986). However, there is little evidence
that P. brassicae infects oilseed
rape systemically; the disease spreads up plants through secondary spore
dispersal and through extension of stems with infected meristematic tissue
(Paul & Rawlinson, 1992).
P. brassicae has a
biotrophic phase after infection followed by a necrotrophic phase (Ashby,
1997), whereas L. maculans has a
necrotrophic phase on leaves followed by a systemic biotrophic phase in petioles. P.
brassicae generally produces secondary spores (conidia) on living leaves,
stems, flowers or pods before tissues die and symptoms appear (Paul &
Rawlinson, 1992). However, L. maculans
generally kills tissues and produces lesions on leaves or stems before conidia
are produced in pycnidia in the dead tissue. Thus the latent period (between
infection and sporulation) of P.
brassicae (c. 200 degree-days;
Figueroa et al., 1995) is shorter than
the incubation period (between infection and symptom appearance), whereas the
incubation period of L. maculans (c. 150 degree-days for leaf lesions;
Biddulph et al., 1998; >200 days
for stem lesions; Hammond & Lewis, 1986) is shorter than the latent period.
Furthermore, the splash-dispersed conidia of P. brassicae have an important role in the secondary spread of
light leaf spot (McCartney & Lacey, 1990), whereas there is no evidence
that the splash-dispersed conidia of L.
maculans have a role in spreading the disease in the UK (Hammond &
Lewis, 1986). However, the extended period of L. maculans ascospore dispersal means that basal stem canker
lesions result from phoma leaf spots on leaves produced at the rosette stage of
winter oilseed rape growth in autumn and winter, whereas upper stem lesions
result from leaf spots on later leaves.
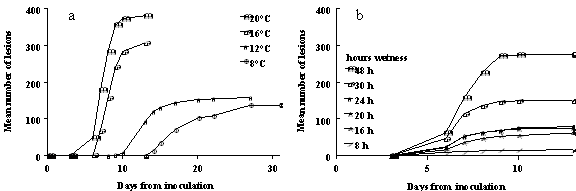
Fig. 2. Effects of temperature and wetness duration
after inoculation on development of phoma leaf spot lesions on oilseed rape
leaves (cv. Nickel) inoculated with
ascospores of Leptosphaeria maculans. Changes with time in number of lesions on 6
plants (4 leaves per plant); a) at different temperature, with 48 h leaf
wetness duration; b) at 20° C with different leaf wetness durations
Evidence
from experiments in which fungicides have been used to manipulate development
of epidemics suggests that light leaf spot generally decreases yields through
killing leaves, and sometimes plants, in winter (Rawlinson et al., 1978; Fitt et al.,
1998a), although occasionally flowers and pods are damaged later. By contrast, stem canker decreases yield
through damage to stems in spring; when basal cankers or upper stem lesions
girdle and kill stems, plants die prematurely and pod and seed development
stops (Sansford et al., 1996). Light leaf spot can be particularly damaging
in long, cool winters because P.
brassicae can continue to infect leaves and sporulate at low temperatures
when oilseed rape growth is very slow (Fitt et
al., 1998a). However, warm winter
weather favours the development of severe stem canker epidemics because L. maculans, with a higher optimum
temperature than P. brassicae, can
then grow rapidly down petioles to infect stems (Hammond & Lewis, 1986).
FORECASTING SEVERITY OF
LIGHT LEAF SPOT AND STEM CANKER
A scheme
for forecasting the severity of light leaf spot epidemics (Fitt et al., 1996; Welham et al., 1998) involves regional risk and
crop risk forecasts at the beginning of the growing season in October, combined
with a protocol for sampling crops to confirm the presence of light leaf
spot. Seasonal, regional risk indices,
predicting the % crops in a region with light leaf spot in the following March
(Fig. 3a), have now been issued in October 1996, 1997 and 1998. In 1998, the forecast was issued as a map showing
the risks in different regions of the UK and placed on the world-wide-web (URL http://www.res.bbsrc.ac.uk/molbio/Lls/), so that growers
could access it. These regional
forecasts are based on survey data collected in the July before they are issued
in October. Regional forecasts can now be updated twice during the
autumn/winter (in January and February) by addition of factors dependent on
autumn rainfall and winter temperature (deviations from 30-year mean values).
Spring disease survey data (i.e. March 1997 and 1998) have been used to
validate predictions made the previous autumn (i.e. October 1996 and
1997). Observed light leaf spot
incidence in spring was never greater than that predicted for a region but was
sometimes considerably smaller, perhaps because many crops had been sprayed
with fungicide. There is further scope
for improving the updating of regional forecasts during the autumn/winter by
incorporating factors relating to results of the autumn disease survey and to
autumn fungicide use to modify the predicted risk of severe light leaf spot.
Regional forecasts have not yet been developed for stem canker, although survey
data show that the greatest risks consistently occur in the east and south of
England. Furthermore, analyses on
eastern region survey data for the period since 1976/77 suggest that rainfall
in August/September greatly influences the risk of severe stem canker epidemics
(Gladders & Symonds, 1995).
Ultimately, there needs to be a forecasting scheme which predicts the
risks of severe epidemics of both light leaf spot and stem canker in each
region.
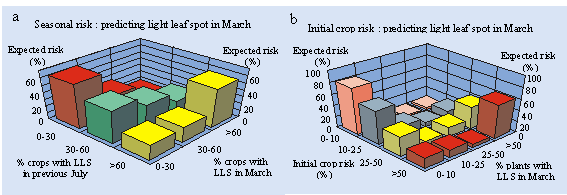
Fig. 3. Prediction of light
leaf spot risk in October for the following March: a) regional risk, based on
survey data from the previous July (% crops in a region with light leaf spot);
b) crop risk, based on sowing date, cultivar and ADAS region (Fitt et al., 1996)
Survey
data has also been used to investigate how factors, such as cultivar, sowing
date and proximity to previous oilseed rape crops, modify the regional risk
index to produce specific crop risk indices for individual crops in October
(Fitt et al., 1996; Fig. 3b). The aim of these crop risk indices is to
provide growers with information about the risks of severe light leaf spot
epidemics in their own crops. However,
variation in survey data for individual crops has made it more difficult to
develop accurate crop risk indices than to develop regional risk indices. Ultimately, there is a need for crop risk
indices that can be updated by using information about local weather (e.g.
occurrence of infection conditions) and fungicide use and encompass risks of
both light leaf spot and stem canker. This information could be incorporated
into an interactive world-wide-web site, so that growers can input information themselves
to derive modified crop risk indices.
To
confirm the presence of light leaf spot in crops with a high predicted risk, a
protocol has been developed for sampling crops (which are frequently
symptomless in autumn when decisions need to be made) to assess the incidence
of the disease (Fitt et al.,
1998a). It is suggested that crops are
inspected at monthly intervals from October to April, looking for patches of
light leaf spot, and sampled by collecting five groups of 20 plants along a
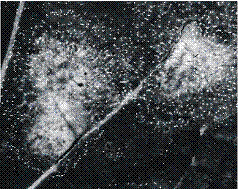
diagonal across them. After incubation
in polyethylene bags at 10 – 15°C for 3 – 4 days, leaves can be inspected for
the presence of the diagnostic white spore pustules of P. brassicae (Fig. 4a) to confirm the presence of the
pathogen. To assess the incidence of the
phoma leaf spot phase of stem canker in crops in the autumn, it is not
necessary to sample or incubate plants since the distinctive spots are clearly
visible in the field, from October onwards (Gladders & Musa, 1980). During
the period between October and April, it is generally sufficient to assess the
% plants with light leaf spot rather than make more time-consuming assessments
of light leaf spot severity (Fitt et al.,
1998b). Similarly, it should only be necessary to assess the % plants with
phoma leaf spot; in theory, one leaf spot can cause one stem lesion/canker of
this monocyclic disease and one stem lesion can kill the plant prematurely.
To
facilitate accurate diagnosis of light leaf spot when plants are symptomless or
display ambiguous symptoms, a molecular method for identification of P. brassicae in infected plants, based
on polymerase chain reaction (PCR) amplification to produce a specific 750
base-pair product, has been developed (Foster et al., 1998; Fig. 4b). This product was produced by all P. brassicae isolates tested and by
infected leaves, but not by healthy leaves or by other oilseed rape
pathogens. However, this diagnostic
method can be used only if expensive PCR equipment is available. Further work is being done using molecular
methods to identify specific proteins produced in infected leaves as a basis
for developing an immunodiagnostic method which can be used by growers. Both molecular and immunodiagnostic methods
have also been developed for identification of A-group and B-group isolates of L. maculans in infected plants since
composition of the L. maculans
population in crops in the autumn affects the risk of severe epidemics
(Williams & Fitt, 1999). However,
accurate assessment of the incidence of light leaf spot or phoma leaf spot in
crops in the autumn depends on the development of accurate methods for sampling
plants in crops, based on a knowledge of the spatial distribution of the
diseases (Hughes et al., 1996). Further work is also required to relate the
observed incidence of light leaf spot or phoma leaf spot in
b
autumn to the severity of light leaf spot or stem canker epidemics in
spring and, ultimately, to yield 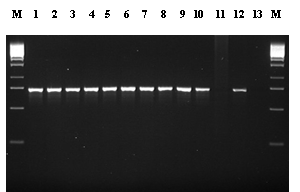
![]()
loss.
Fig. 4. Diagnosis of infection by Pyrenopeziza brassicae (light leaf spot) in winter oilseed rape leaves: a) lesions surrounded by characteristic white spore pustules, produced after incubation in a polyethylene bag (Fitt et al., 1998a); b) diagnostic 750 base-pair product produced in a polymerase chain reaction (PCR) using primers specific for P. brassicae (Foster et al., 1998). Lane M, DNA size marker; lanes 1-10, P. brassicae isolates; lane 11, healthy oilseed rape leaf; lane 12, oilseed rape leaf with light leaf spot symptoms; lane 13, negative control
DISCUSSION: INTEGRATED DISEASE MANAGEMENT
A strategy for integrated management of diseases on winter oilseed rape in the UK currently needs to rely on use of fungicides, since there is no complete resistance to stem canker, light leaf spot, stem rot or dark pod spot in any current cultivars. Cultural practices, such as ploughing in of debris and volunteers from previous oilseed rape crops before emergence of new crops, can help to decrease inoculum sources. Furthermore, there are differences between cultivars in their level of resistance to stem canker and light leaf spot under field conditions (Anonymous, 1997) and growers can select cultivars which are most resistant to the disease which is predominant in their area. More resistant cultivars are likely to require less fungicide treatment; e.g. tebuconazole increased yields of cv. Capitol (rating 8 for resistance to light leaf spot) less than those of cv. Bristol (resistance rating 2) in experiments at Rothamsted where severe light leaf spot developed (Sutherland et al., 1998). Nevertheless, the estimates of losses from diseases of winter oilseed rape (Fig. 1), despite expenditure of up to £12M per season on fungicides (Turner et al., 1999), suggest that there is considerable scope to improve timing of fungicide applications. The survey data suggest that many crops which warranted treatment against stem canker or light leaf spot were not sprayed in autumn and that other crops were sprayed unnecessarily against stem rot or dark pod spot in spring. The results of spray timing experiments and information about the epidemiology of these diseases in the UK suggest that there are three main periods in the growing season when spray decisions need to be made.
1. Autumn. In autumn, decisions need to be taken about application of fungicides to prevent spread of L. maculans from phoma leaf spots to the stem (stem canker) and to eradicate symptomless infections by P. brassicae (light leaf spot) (Fig. 5). The timing of the spray against the phoma leaf spot phase of stem canker is critical, since the pathogen cannot be controlled once it has spread from the leaf to the stem. Poor control has been achieved by sprays applied too early in some years, or too late in others (Gladders et al., 1998). Whilst survey results already indicate that the greatest risks of severe stem canker epidemics are in the east and south, there is a need to develop regional forecasts to quantify the risks in each region more accurately. To improve the timing of sprays against stem canker, it is necessary to develop methods for predicting the time when incidence of phoma leaf spot increases rapidly (which varies between seasons and sites from early October to late November; Biddulph et al., pers. comm.). Recent research has shown that this increase in incidence of phoma leaf spot cannot easily be related to peaks in numbers of air-borne L. maculans ascospores (West et al., 1998) or to occurrence of weather favourable for infection (Biddulph et al., 1998). It seems likely that, as in France (Pérès & Poisson, 1997), differences between seasons and sites in timing of leaf spotting may be related to differences in factors affecting maturation of ascospores, such as rainfall in August/September (Gladders & Symonds, 1995). Thus, there is a need to test whether the weather-based CETIOM model (Pérès & Poisson, 1997) for describing ascospore maturation can be used to improve timing of sprays against stem canker in the UK.
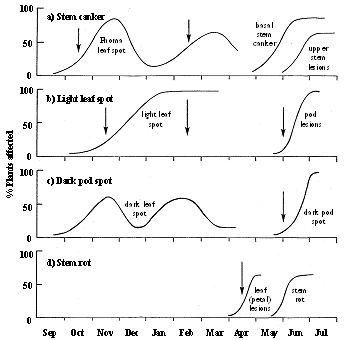
Fig. 5. Times (arrows) at which decisions need to made about application of fungicides to UK winter oilseed rape, in relation to typical disease progress curves of: a) stem canker (Leptosphaeria maculans); b) light leaf spot (Pyrenopeziza brassicae); c) stem rot (Sclerotinia sclerotiorum); d) dark pod spot (Alternaria brassicae). Modified from Fitt et al. (1997)
In autumn, growers in Scotland and the north and west of England need to be more concerned about the risk of severe light leaf spot epidemics than about stem canker. The regional light leaf spot forecasts can help to guide their decisions (Welham et al., 1998). There is a need to extend these regional forecasts to Scotland and to introduce crop risk forecasts in a form that can be used by growers. The optimum timing for spraying against the phoma leaf spot phase of stem canker is often in October but for spraying against light leaf spot it is often in November. Growers in areas at risk from both diseases may need to compromise their spray timing, although the timing of sprays against stem canker is often more critical than timing of sprays against light leaf spot (Gladders et al., 1998; Sansford et al., 1996).
2. Late winter/early spring (stem extension). There is a need to reassess the risk of severe stem canker or light leaf spot in late winter/early spring, since severe epidemics of either disease cannot be controlled by a single fungicide spray (Fitt et al., 1997). Generally, two half-dose applications of fungicide, in autumn and late winter, provide better disease control and produce better yield responses than a single full-dose spray in autumn or late winter. The second spray provides control of the phoma leaf spots which produce the upper stem lesions and against an increase in epidemic development of the polycyclic light leaf spot. The yield loss model of Su et al. (1998), based on % plants with light leaf spot in the crop, can be used to assess the risk of severe light leaf spot at this stage and observation of phoma leaf spotting can help decisions about a further spray against stem canker. The updated regional light leaf spot forecasts, based on winter weather, can also be considered, but there is a need to develop reliable regional risk and crop risk forecasts, combined for light leaf spot and stem canker, which can be updated in the late winter/early spring.
3. Spring (flowering). A single, well-timed spray during flowering (GS 4,5) can prevent the establishment of Sclerotinia sclerotiorum in fallen petals on leaves and development of stem rot (Davies, 1995). Methods developed for assessing the risk of severe stem rot epidemics, based on previous incidence of stem rot, were greatly improved by applying a scheme for culturing fallen petals sampled from crops to assess the presence of S. sclerotiorum. These methods confirmed that many crops are sprayed unnecessarily against stem rot, since the coincidence of petal fall, S. sclerotiorum ascospore release and wet weather required for stem rot development rarely occurs in the UK. Similarly, single post-flowering (95% petal fall) sprays can provide good control of dark pod spot (Gladders, 1988) and the pod phase of light leaf spot but are rarely necessary in the UK. Since lodging favours the development of dark pod spot, avoidance of excessive seed rates at sowing or excessive rates of nitrogen fertiliser can help to minimise risk of dark pod spot epidemics. Decisions about the need for control of dark pod spot can be based on observations of disease in the crop and occurrence of weather favourable for dark pod spot development in May (Hong et al., 1996; Kennedy et al., 1995).
Surveys suggest that the incidence of light leaf spot in England and Wales has decreased since 1995 (Fig. 1), as the proportion of crops sprayed in autumn has increased (Turner, pers. comm.), suggesting that the light leaf spot forecasting scheme has helped to improve farming practice. However, there is still much scope for work to improve timing of sprays against stem canker, and to decrease unnecessary application of sprays against stem rot or dark pod spot. Ultimately, there is a need to construct a decision support system for integrated management of diseases in winter oilseed rape in the UK. However, such a decision support system can be reliable and robust only if it is based on accurate understanding and accurate models of the epidemiology of the important diseases. The priorities now must be to obtain accurate biological data about the development of stem canker, to construct accurate models to describe these data, and to develop combined regional risk and crop risk forecasts for light leaf spot and stem canker.
ACKNOWLEDGEMENTS
This
work was funded by the UK Ministry of Agriculture, Fisheries and Food, the
Biotechnology and Biological Sciences Research Council, the Home-Grown Cereals
Authority, the Scottish Office, the European Union, the Perry Foundation and
Bayer PLC. We thank J.E. Biddulph, N. Castells‑Brooke, S.J. Elcock,
S.J. Foster, T. Gilles, P.K. Leech, C.E. Sansford, S. Souter, J.M. Steed, B.V.
Symonds, J.S. West and other colleagues for their contribution to the work.
REFERENCES
Anonymous
(1997). UK recommended list of oilseeds
1997. National Institute of Agricultural
Botany, Cambridge. 7pp.
Ansan-Melayah, D., Rouxel, T., Bertrandy, J.,
Letarnec, B., Mendes-Pereira, E. & Balesdent, M.H. (1997). Field efficiency of Brassica napus specific resistance correlates with Leptosphaeria maculans population
structure. European Journal of Plant Pathology 103, 835-841.
Ashby, A.M. (1997). A molecular view through the
looking glass: the Pyrenopeziza brassicae
– Brassica interaction. Advances
in Botanical Research 24, 32-70
Biddulph, J.E., Fitt, B.D.L. & Welham, S.J.
(1998). Effects of temperature and
wetness duration on infection of oilseed rape leaves by ascospores of Leptosphaeria maculans (stem
canker). Abstract 2.5.13, 7th International Congress of Plant
Pathology, Edinburgh.
Davies, J.M.L. (1995). Petal culturing to forecast Sclerotinia in winter oilseed rape. Proceedings of 9th International Rapeseed
Congress, Cambridge, 1011-1012.
Figueroa, L., Shaw, M.W., Fitt, B.D.L.,
McCartney, H.A. & Welham, S.J. (1994).
Effects of previous cropping and fungicide timing on the development of
light leaf spot (Pyrenopeziza brassicae),
seed yield and quality of winter oilseed rape (Brassica napus). Annals of Applied Biology 124, 221-239.
Figueroa, L., Fitt, B.D.L., Welham, S.J., Shaw,
M.W. & McCartney, H.A. (1995). Early
development of light leaf spot (Pyrenopeziza
brassicae) on winter oilseed rape (Brassica
napus) in relation to temperature and leaf wetness. Plant Pathology 44, 641‑654.
Fitt, B.D.L., Gladders, P., Turner, J.A.,
Sutherland, K.G. & Welham, S.J. (1996).
Predicting risk of severe light leaf spot (Pyrenopeziza brassicae) on winter oilseed rape in the UK. 1996
Brighton Crop Protection Conference – Pests and Diseases, 239-244
Fitt, B.D.L., Gladders, P., Turner, J.A.,
Sutherland, K.G., Welham, S.J. & Davies, J.M.L. (1997). Prospects for developing a forecasting scheme
to optimise use of fungicides for disease control on winter oilseed rape in the
UK. Aspects
of Applied Biology 48, 135-142.
Fitt, B.D.L., Doughty, K.J., Gladders, P., Steed, J.M.
& Sutherland, K.G. (1998a). Diagnosis of light leaf spot (Pyrenopeziza brassicae) on winter
oilseed rape (Brassica napus) in the
UK. Annals of Applied Biology 133,
155-166.
Fitt, B.D.L., Doughty, K.J., Gilles, T., Gladders,
P., Steed, J.M., Su, H. & Sutherland, K.G. (1998b). Methods for assessment of light leaf spot (Pyrenopeziza brassicae) on winter
oilseed rape (Brassica napus) in the
UK. Annals of Applied Biology 133,
329-342.
Foster, S.J., Ashby, A.M. & Fitt, B.D.L. (1998).
Molecular diagnosis of light leaf spot (Pyrenopeziza
brassicae) on winter oilseed rape. IOBC Bulletin 21, 235-239.
Gladders, P. (1988).
The contribution and value of pesticides to disease control in
combinable break crops. In: Control of Plant Diseases: Costs and
Benefits, pp. 29-50. Eds. B.C.
Clifford & E. Lester. Oxford:
Blackwell Scientific Publications.
Gladders, P. & Musa, T.M. (1980). Observations
on the epidemiology of Leptosphaeria
maculans stem canker in winter oilseed rape. Plant
Pathology 44, 641-654.
Gladders, P. & Symonds, B.V. (1995). Occurrence of canker (Leptosphaeria maculans) in winter oilseed rape in Eastern England,
1977-1993. IOBC Bulletin 18, 1-11.
Gladders, P., Symonds, B.V., Hardwick, N.V. &
Sansford C.E. (1998). Opportunities to
control canker (Leptosphaeria maculans)
in winter oilseed rape by improving spray timing. IOBC Bulletin 21, 111‑120.
Hammond, K.E. & Lewis, B.G. (1986). The timing
and sequence of events leading to stem canker disease in populations of Brassica napus var. oleifera in the field. Plant
Pathology 35, 551-564.
Hammond, K.E., Lewis, B.G. & Musa, T.M.
(1985). A systemic pathway in the
infection of oilseed rape plants by Leptosphaeria
maculans. Plant Pathology 34,
557-565.
Hardwick, N.V. & Turner, J.A. (1994). Fungicide use on winter oilseed rape in
England and Wales, 1986-1993. 4th International Conference on Plant
Diseases, Bordeaux. ANPP, 1163-1170.
Hong, C.X., Fitt, B.D.L.& Welham, S.J.
(1966). Effects of wetness period and
temperature on development of dark pod spot (Alternaria brassicae) on oilseed rape (Brassica napus). Plant
Pathology 45, 1077-1089.
Hughes, G. Madden, L.V.& Munkvold, G.P.
(1996). Cluster sampling for disease
incidence data. Phytopathology 86,
132-137.
Kennedy, R. & Graham, A.M. (1995). Infection of oilseed rape by Alternaria brassicae under varying
conditions of temperatures and wetness. Proceedings of 9th International Rapeseed
Congress, Cambridge, 601-603.
McCartney, H.A. & Lacey, M.E. (1990). The
production and release of ascospores of Pyrenopeziza
brassicae on oilseed rape. Plant Pathology 39, 17-32.
Majer, D., Lewis, B.G. & Mithen, R. (1998). Genetic variation among field isolates of Pyrenopeziza brassicae. Plant
Pathology 47, 22-28.
Paul, V.H.& Rawlinson, C.J. (1992). Diseases
and Pests of Rape. Verlag Th. Mann,
Gelsenkirchen-Buer, Germany. 132 pp.
Pérès, A. & Poisson, B. (1997). Phoma du colza: avancées en
epidemiologie. CETIOM – Oléoscope 40,
37-40.
Rawlinson, C.J., Muthyalu, G., Cayley, G.R.
(1984). Fungicide effects on light leaf
spot, canker, crop growth and yield of winter oilseed rape. Journal
of Agricultural Science, Cambridge 103, 613-628.
Rawlinson, C.J., Sutton, B.C. & Muthyalu, G. (1978). Taxonomy and biology of Pyrenopeziza
brassicae sp. nov. (Cylindrosporium
concentricum), a pathogen of winter oilseed rape (Brassica napus ssp. oleifera). Transactions
of the British Mycological Society 71, 425-439.
Sansford, C.E., Fitt, B.D.L., Gladders, P.,
Lockley, K.D. & Sutherland, K.G. (1996). Oilseed rape: disease development,
forecasting and yield loss relationships.
Home-Grown Cereals Authority Project Report OS 17. 185 pp.
Su, H., Fitt, B.D.L., Welham, S.J., Sansford,
C.E. & Sutherland, K.G.(1998). Effects of light leaf spot (Pyrenopeziza brassicae) on yield of
winter oilseed rape (Brassica napus).
Annals of Applied Biology 132, 371-386.
Sutherland, K.G., Fitt, B.D.L., Steed, J.M.,
Welham, S.J., Gladders, P. & Turner, J.A. (1998). Development and control of light leaf spot (Pyrenopeziza brassicae) epidemics in
winter oilseed rape in the UK. 1998 Brighton Crop Protection Conference –
Pests and Diseases, 1053-1058.
Sutherland, K.G., Wale, S.J., Sansford, C.E.
(1995). Effect of different epidemics of
Pyrenopeziza brassicae on yield loss
in winter oilseed rape. Proceedings 9th International Rapeseed Congress, Cambridge, 1004-1006.
Sylvester-Bradley, R. & Makepeace, R.J.
(1985). Revision of a code for stages of
development in oilseed rape (Brassica
napus L.). Aspects of Applied Biology
10, 395-400.
Turner, J.A., Gladders, P., Elcock, S.J., Walters,
K.F.A.& Wright, D.M. (1999). Winter oilseed rape: survey of pests and
diseases, 1997/98. CSL/ADAS Research and Development, York. 57 pp.
Welham, S.J., Turner, J.A., Fitt, B.D.L.,
Gladders, P. & Sutherland, K.G. (1998). Empirical models for prediction of
regional light leaf spot (Pyrenopeziza
brassicae) incidence on winter oilseed rape in the UK. Abstract 2.1.9, 7th International Congress of Plant
Pathology, Edinburgh.
West, J., Leech, P. & Fitt, B.D.L. (1998). The effects of rainfall on numbers of air-borne ascospores of Leptosphaeria maculans and the development of phoma leaf lesions on winter oilseed rape. Abstract 2.1.7, 7th International Congress of Plant Pathology, Edinburgh.
Williams,
R.H. & Fitt, B.D.L. (1999).
Differentiating A and B groups of Leptosphaeria
maculans, causal agent of stem canker of winter oilseed rape in the UK – a
review. Plant Pathology 46 (in
press).