INHERITANCE OF ERUCIC ACID CONTENT IN
YELLOW MUSTARD (SINAPIS ALBA L.)
Wilhelmina J. Drost 1,2, Gerhard Rakow1, and Philip Raney1
1Agriculture and Agri-Food Canada, Saskatoon Research Centre
107 Science Place, Saskatoon, SK, Canada, S7N 0X2
2Department of Plant Sciences, University of Saskatchewan
51 Campus Drive, Saskatoon, SK, Canada, S7N 5A8
ABSTRACT
The inheritance of erucic acid content (C22:1) in S. alba was studied in F1, BC1F1, and F2 progeny of a cross between the high erucic acid variety Sabre (>50% C22:1) and the low erucic acid line WD96-2 (<0.1% C22:1). Segregation patterns in BC1F1 and F2 seed indicated that erucic acid content in S. alba was controlled by a single gene exhibiting partial dominance of high over low erucic acid contents.
KEYWORDS
canola-quality oil, fatty acid
INTRODUCTION
Yellow mustard (S. alba) has the potential to be a major oilseed crop for Canada. It has many advantages over the currently grown canola species Brassica napus and B. rapa including heat and drought tolerance, resistance to blackspot (Alternaria brassicae Berk. Sacc) (Brun et al. 1987), and tolerance to flea beetle attack (Phyllotreta cruciferae Goeze)(Bodnaryk and Lamb 1991). Other desirable characteristics of S. alba include early maturity, pod shatter resistance, large seed size allowing deeper seeding of the crop compared to shallow seeding of the Brassica species (Brandt 1992), and yellow seeds in which chlorophyll levels are not masked in immature seed. Currently, S. alba is grown for condiment mustard purposes in western Canada and its seed oil contains high levels of erucic acid while canola-quality oil requires erucic acid content to be less than 2%. Low erucic acid, low glucosinolate forms of S. alba have been developed at the AAFC Saskatoon Research Centre (Raney et al. 1995). The objective of this study was to determine the inheritance of erucic acid content in yellow mustard which will assist in developing strategies for the breeding of canola-quality S. alba varieties.
MATERIALS AND METHODS
Parents for the study of erucic acid content inheritance in S. alba were the high erucic acid variety Sabre and the low erucic acid line WD96-2. Both genotypes were developed at the AAFC Saskatoon Research Centre.
F1, BC1F1, and F2 populations were developed using bud pollination techniques in a controlled environment. F1 seed was produced by reciprocally crossing Sabre with WD96-2. BC1F1 seed was produced by reciprocally crossing F1 plants with each parent. All plants were grown from half-seeds (Downey and Harvey 1963) and analysed for fatty acid composition by the gas chromatographic method of Thies (1971) with minor modifications.
RESULTS AND DISCUSSION
Erucic acid content of Sabre, WD96-2, and F1 seed
The erucic acid content of Sabre was 53.2% and that of WD96-2 was <0.1% (Fig. 1). The erucic acid content of F1 seed was intermediate between that of the parents but was shifted towards the high erucic acid parent (Fig. 1). This indicated embryonic control of erucic acid content with partial dominance of high over low erucic acid contents. The erucic acid content of F1 seed borne on Sabre (39.5%) was higher than that of F1 seed borne on WD96-2 (36.2%).
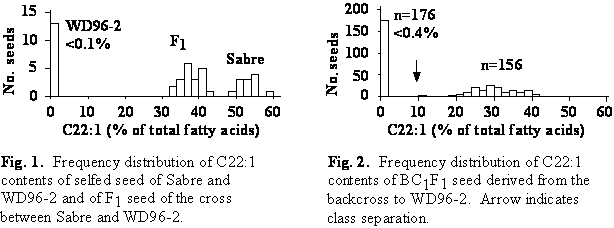 |
Erucic acid content of BC1F1 seed derived from the backcross to WD96-2
The erucic acid content of seed derived from the backcross to WD96-2 ranged from <0.1 to 41.4% (Fig. 2). The frequency distribution segregated into two classes: seed with low (<0.4%) and intermediate (10-42%) erucic acid content. A 1:1 segregation ratio indicative of a one gene model was not significantly different from the observed segregation ratios in each of the four reciprocal backcross populations.
Erucic acid content of BC1F1 seed derived from the backcross to Sabre
The erucic acid content ranged from 30.4 to 56.6% in seed derived from the backcross to Sabre (Fig. 3). The frequency distribution segregated into two classes: seed with intermediate (30-44%) and high (>44%) erucic acid content. The expected segregation ratio of 1:1 was significantly different from the observed segregation ratio in the BC1F1 population carrying cytoplasm of the low erucic acid parent WD96-2.
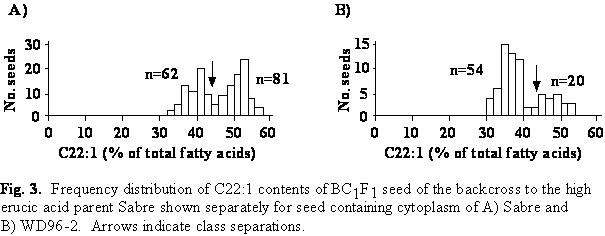 |
Erucic acid content of F2 seed
The erucic acid content of F2 seed ranged from <0.1 to 56.9% (Fig. 4). The frequency distribution of reciprocal F2 populations segregated into three classes: seed with low (<0.4%), intermediate (22-44%) and high (>44%) erucic acid content. The 1:2:1 segregation ratio in accordance with the backcross segregation ratios was tested and was significantly different from the observed ratio in the F2 population carrying the cytoplasm of the low erucic acid parent WD96-2. The numbers of seed with intermediate and high erucic acid contents were combined and the F2 segregation ratio of 1:3 was tested. This theoretical ratio was not significantly different from the observed ratios in either F2 population.
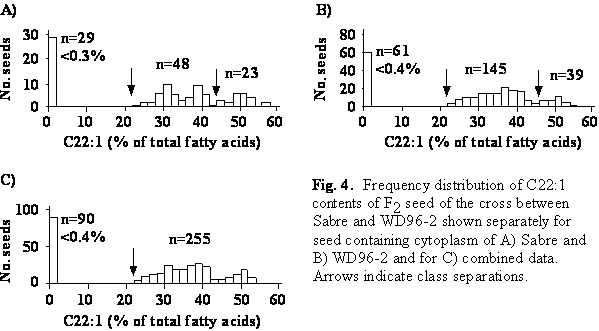 |
CONCLUSIONS
Intermediate levels of erucic acid in F1 seed indicated that erucic acid content in S. alba seed was primarily controlled by the genotype of the embryo with partial dominance of high over low erucic acid contents.
Segregation patterns of erucic acid content of BC1F1 seed derived from the backcross to the low erucic acid parent indicated that erucic acid content in S. alba was controlled by a single gene. The one gene inheritance model was supported by segregation patterns of erucic acid content in BC1F1 seed derived from the backcross to the high erucic acid parent Sabre and in F2 seed. Expected segregation ratios were significantly different from observed ratios in BC1F1 and F2 populations carrying the cytoplasm of WD96-2 which indicated a cytoplasmic effect on erucic acid content.
The results of this study indicated that the low erucic acid trait in S. alba was highly heritable and controlled by a single gene and therefore, could easily be reselected following backcrossing in a breeding program.
ACKNOWLEDGEMENTS
Financial support from the following sources is gratefully acknowledged: Saskatchewan Canola Development Commission, Canada-Saskatchewan Agri-Food Innovation Fund, Canadian Seed Growers’ Association, University of Saskatchewan, Canada.
References
Bodnaryk, R.P., and Lamb, R.J. 1991. Mechanisms of resistance to flea beetle, Phyllotreta cruciferae (Goeze), in mustard seedlings, Sinapis alba L. Can. J. Plant Sci. 71:13-20.
Brandt, S.A. 1992. Depths, rates and dates of seeding and yield of yellow mustard (Sinapis alba L.) Can. J. Plant Sci. 72:351-359.
Brun, H, Plessis, J., and Renard, M. 1987. Resistance of some crucifers to Alternaria brassicae (Berk) Sacc. Proc. 7th Int. Rapeseed Cong., Poznan, Poland, May 11-14, 1987. III:247.
Downey, R.K. and Harvey, B.L. 1963. Methods of breeding for oil quality in rape. Can. J. Plant Sci. 43:271-275.
Raney, P., Rakow, G., and Olson, T. 1995. Development of low erucic, low glucosinolate Sinapis alba. Proc. 9th Int. Rapeseed Cong. Cambridge, UK, Vol. 2:416-418.
Thies, W. 1971. Rapid and simple analysis of fatty acid composition of individual rape cotyledons. 1. Gas and paper chromatographic techniques. Z. Pflanzenzhchtg 65:181-202.