low linolenic acid, ‘zero’ aliphatic glucosinolate Brassica napus
J Philip Raney, Gerhard FW Rakow, Richard K Gugel
and Todd V Olson
Agriculture and Agri-Food Canada, Saskatoon Research Centre, 107 Science Place, Saskatoon, SK, Canada, S7N 0X2
ABSTRACT
‘Zero’ aliphatic glucosinolate resynthesized Brassica napus germplasm has been developed at the Saskatoon Research Centre from interspecific crosses with B. rapa and B. oleracea. To improve the oil content, oil quality and agronomic performance of this germplasm, a ‘zero’ aliphatic glucosinolate (<1.0 mmole/g seed) line was crossed with a low linolenic acid (2%) B. napus line. The F1 of this cross was then crossed with a high oil content, blackleg resistant B. napus line. The F1 progeny of this three-way cross was then used for doubled haploid production via microspore culture, or selfed and successive generations selected for phenotypes combining the traits of the parents. Low linolenic acid phenotypes were first selected on the basis of the fatty acid profiles of half-seeds. Plants of selected half-seeds were then grown in a greenhouse, selfed and screened for resistance to blackleg. The seed harvested from these plants was then used for glucosinolate content evaluation and confirmation of fatty acid composition. This process was repeated on plants deemed to have the most favourable combination of these traits. The results of the first field trials of selected lines and the doubled haploid progeny are discussed.
KEYWORDS: fatty acid composition, half-seed selection, blackleg, oil content, seed colour
INTRODUCTION
Brassica napus canola is Canada's major edible oilseed crop. Its value is limited by the value of the derived oil and meal. The major determinant in the value of canola oil is the fatty acid composition of the seed oil. Polyunsaturated fatty acids such as linolenic acid destabilize the oil. The linolenic acid content of standard canola cultivars is significantly above human nutritional requirements. It can be reduced to 2-3% of the total fatty acid content of the oil to enhance stability and reduce hydrogenation times, and yet still satisfy nutritional requirements. Several studies on recently developed low linolenic acid lines of canola have demonstrated that this type of canola oil has improved storage and frying stability compared with standard canola types (Eskin et al. 1989, Prevot et al. 1990, Przybylski et al. 1993). Major determinants in the value of canola meal are the levels of antinutritive compounds such as glucosinolates, and protein content and metabolizable energy levels that are limited in part by the fibre content of the meal. The antinutritive effects of glucosinolates have been established (Bell 1984, Bell et al. 1991). Breeding for the lowest possible glucosinolate content in canola meal should enhance its use as an animal feed (Bell 1993). Lighter coloured seed generally has lower fibre content (Rashid 1995); therefore, it is desirable for canola to become a yellow seeded product. Canada's B. rapa canola crop has already begun this transition.
Producers can experience significant yield losses in B. napus due to blackleg disease caused by Leptosphaeria maculans (Gugel and Petrie 1992). The introduction of strong blackleg resistance into B. napus canola should help to stabilize canola yields for Canadian producers.
In recent years biotechnological approaches to plant breeding have become increasingly popular. Microspore culture in particular is a useful tool for the efficient and rapid production of inbreds in B. napus (Lichter 1982, Polsoni et al. 1988). This system of plant regeneration may be particularly useful for rare genotype selection because of the simplified genetics in a haploid system (Siebel and Pauls 1989).
Recently, ‘zero’ aliphatic glucosinolate resynthesized B. napus germplasm with an aliphatic glucosinolate content of less than one µmole per gram seed was developed at the Saskatoon Research Centre from interspecific crosses with B. rapa and B. alboglabra (Raney et al. 1995). This paper discusses our effort to improve the oil content, oil quality and agronomic performance of this germplasm through crosses with low linolenic acid B. napus germplasm, and with elite high yielding, high oil content, blackleg resistant B. napus germplasm.
MATERIALS AND METHODS
Parental materials and selections
The ‘zero’ aliphatic glucosinolate (<1.0 mmole/g seed) parent RSYN1-43 was derived from interspecific crosses between the yellow seeded B. napus line YN90-1016, the yellow seeded B. rapa cultivar AC Parkland and light brown seeded B. alboglabra. The yellow seeded, low linolenic acid (3%) parent TO95-1299 was derived from a cross between the low linolenic acid B. napus cultivar Apollo and YN90-1016. The high oil content, blackleg resistant parent N93-1526 was derived from a cross between an adapted B. napus canola line and Shiralee, a highly blackleg resistant B. napus cultivar from Australia. Reciprocal crosses were made between RSYN1-43 and TO95-1299. The F1 of this cross was then crossed with N93-1526. The F1 progeny of this three-way cross was then used for doubled haploid (DH) production via microspore culture and/or selfed and the successive generations selected for phenotypes combining the above mentioned traits of the parents. Over 400 DH lines were generated and 244 of these evaluated in the field in either 1997 or 1998; 79 lines were evaluated in both years.
The inbreeding selection program began by selecting putative low linolenic acid F2 progeny by half-seed analysis (Downey and Harvey 1963). Twenty-four F2 seeds of each of 33 F1 lines were analyzed and 67 were selected for continuation. On the basis of the segregation of half-seed fatty acid profiles from each line, 10 lines were selected as having the best chance to produce low linolenic acid progeny. These lines were evaluated in a blackleg disease nursery in 1996, where 1,608 of 7,000 seeds planted reached maturity and were rated for blackleg disease reaction. A total of 106 plants were rated as resistant to blackleg; these were then harvested and rated for glucosinolate content and seed colour. Thirty-three lines were continued by half-seed analysis (48 seeds from each), and 247 F3 seeds were selected and grown in a greenhouse. The plants were concurrently selfed and screened for blackleg resistance; of 232 plants harvested, 127 were rated as resistant or intermediate. Seed from these plants was analyzed for fatty acid composition, glucosinolate content and seed colour. Twenty-three lines were selected for continuation by half-seed analysis (24 seeds of each); 260 selected F4 seeds were grown in a greenhouse and concurrently selfed and screened for blackleg resistance. F5 seed from 150 plants was harvested and planted in a field nursery and a blackleg disease nursery in 1998. Also, 46 F4 lines were planted in the 1998 field nurseries. Seed from the harvested rows was evaluated for seed quality traits.
Analytical Methods
The fatty acid composition of bulk seed samples and germinated half-seeds was determined by gas chromatography of the methyl esters (Thies 1971, Raney et al. 1995). The glucosinolate content of seed was determined by gas chromatography of the trimethylsilyl derivatives of the desulphoglucosinolates (Thies 1980, Raney et al. 1995). Aliphatic glucosinolate content is reported as the sum in µmoles/g seed of allyl, 3-butenyl, 4-pentenyl, 2-hydroxy-3-butenyl and 2-hydroxy-4-pentenyl glucosinolates. Oil content of harvested nursery rows was measured on dried, intact seed using a continuous-wave, low-resolution nuclear magnetic resonance instrument. Seed colour (Whiteness Index, ASTM Method E313) was measured with a reflectance colorimeter. The field nurseries consisted of single rows three metres long with two replicates. The susceptibility of lines to blackleg was evaluated in irrigated field disease nurseries under conditions conducive to the disease (Rimmer et al. 1995). Disease severity was assessed when the plants were beginning to ripen by scoring individual plants on a 0 (plant healthy) to 5 (plant dead) scale based on the severity of blackleg symptoms (R. Gugel, unpublished). The blackleg reaction of individual plants was also rated in the greenhouse by inoculation of basal stems at bolting with a highly virulent isolate of L. maculans, and rating the severity of infection at harvest (Gugel et al. 1990). Plants were rated as resistant, having an intermediate reaction, or as susceptible.
RESULTS AND DISCUSSION
Diploid progeny and inbred F4 and F5 progeny
The DH progeny rows showed segregation for all the seed quality traits under consideration (Fig. 1) and for blackleg resistance (data not shown). The data from the 1997 nursery was similar: there were correlations of 0.83 for aliphatic glucosinolate, 0.78 for oil content, 0.92 for linolenic acid content, and 0.90 for seed colour between the two nursery years. The inbred progeny demonstrated less segregation for all the seed quality traits and tended toward the desired level of each trait, particularly for aliphatic glucosinolate and linolenic acid contents (Fig. 1). The oil content distribution is interesting in that the DH progeny tended to have lower oil contents than the inbred progeny even though there had been no deliberate oil content selection in either case. This may have been the result of fortuitous selection for higher oil content in the 1996 blackleg nursery. Some DH progeny oil contents were even lower than in the low oil parent (RSYN1-43).
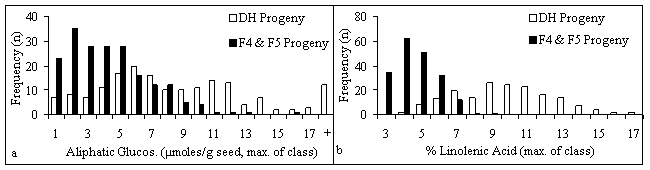
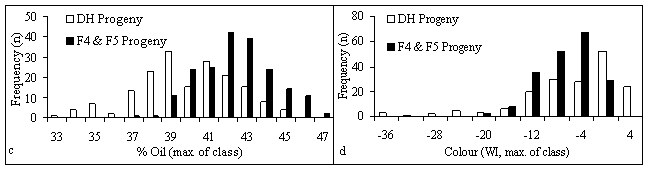
Figure 1. Histograms of seed quality traits in progeny grown in 1998 nursery: a) Aliphatic glucosinolate; b) Linolenic acid content; c) Oil content; d) Seed colour (Whiteness Index)
Best lines and parents
The three parents were evaluated in both years in the field nursery and in the 1998 disease nursery.
The results for 1998 are shown in Table 1; the results for 1997 were very similar. All the best lines in the 1998 nursery were from the inbreeding program (Criteria: <1.0 µmole/g aliphatic glucosinolate, <3.3% linolenic acid, within 3% of the high oil parent and whiteness index <-7). This was mainly due to lower oil and higher linolenic acid contents in the DH progeny. The best lines also had a good blackleg rating in the disease nursery. However, the low level of blackleg disease pressure in 1998 resulted in blackleg data that is less reliable than it could be.
Table 1: Seed quality traits and disease ratings of selected lines from the 1998 field nurseries
Line Generation Coloura Ali Glub % Oil C18:3c Blacklegd
RSYN1-43 Parent -6.2 0.3 36.1 12.4 0.2
TO95-1299 Parent -16.5 6.3 42.5 3.3 0.8
N93-1526 Parent -0.3 8.1 45.0 7.8 0.2
TO97-3239-7 F5 -15.9 0.9 42.7 2.4 0.1
TO97-3233-4 F5 -13.2 1.0 42.3 2.6 0.2
TO97-2387-1 F4 -11.1 0.8 42.8 2.7 0.3
TO97-3233-3 F5 -11.0 0.7 44.3 3.2 0.4
TO97-3233-2 F5 -9.7 0.7 42.4 3.2 0.6
TO97-3232-14 F5 -8.1 1.0 43.8 3.3 0.2
TO97-3233-6 F5 -7.5 0.7 43.8 2.8 0.2
AC Excel Nursery Check 0.9 7.5 44.4 6.9
AC Parkland Nursery Check -35.8 8.6 44.8 11.2
N89-53 Nursery Check 0.9 13.6 45.4 6.1
YN90-1016 Nursery Check -25.6 8.2 40.7 9.4
Quantum Blackleg Nursery Check (Resistant) 0.2
Westar Blackleg Nursery Check (Susceptible) 1.8
a. Whiteness Index. b. Aliphatic glucosinolate. c. Linolenic acid. d. Blackleg severity.
CONCLUSION
The effort to combine the seed quality traits ‘zero’ aliphatic glucosinolate, low linolenic acid and high oil content into a single cultivar is well on its way to success. Several very low aliphatic glucosinolate lines were found that had significantly improved oil content over RSYN1-43 and carried the low linolenic acid trait. These lines also had a light seed colour and were not significantly different from the blackleg resistant cultivar Quantum in their disease reaction. The conventional inbreeding program outperformed the doubled haploid program.
ACKNOWLEDGEMENTS
The authors acknowledge the support of the Canola Council of Canada and the Matching Investments Initiative program of Agriculture and Agri-Food Canada for this project.
REFERENCES
Bell, J.M. 1984. Nutrients and toxicants in rapeseed meal: a review. Journal of Animal Science 58, 996-1000.
Bell, J.M., Keith, M.O., and Hutcheson, D.S. 1991. Nutritional evaluation of very low glucosinolate canola meal. Canadian Journal of Animal Science 71, 497-506.
Bell, J.M. 1993. Factors affecting the nutritional value of canola meal: A review. Canadian Journal of Animal Science 73, 679-697.
Downey, R.K. and Harvey, B.L. 1963. Methods of breeding for oil quality in rape. Canadian Journal of Plant Science 43, 271-275.
Eskin, N.A.M., Vaisey-Genser, M., Durance-Todd, S. and Przybylski, R. 1989. Stability of low linolenic acid canola oil to frying temperatures. Journal of the American Oil Chemists Society 66, 1081-1084.
Gugel, R.K., Séguin-Swartz, G. and Petrie, G.A. 1990. Pathogenicity of three isolates of Leptosphaeria maculans on Brassica species and other crucifers. Canadian Journal of Plant Pathology 12, 75-82.
Gugel, R.K. and Petrie, G.A. 1992. History, occurrence, impact, and control of blackleg of rapeseed. Canadian Journal of Plant Pathology 14, 36-45.
Lichter, R. 1982. Induction of haploid plants from isolated pollen of Brassica napus. Zeischrift für Pflanzenphysiologie 105, 427-434.
Polsoni, L., Kott, L.S. and Beversdorf, W.D. 1988. Large-scale microspore culture technique for mutation-selection studies in Brassica napus. Canadian Journal of Botany 66, 1681-1685.
Prevot, A., Perrin, J.L., Laclaverie, G., Auge, P. and Coustille, J.L. 1990. A new variety of low linolenic rapeseed oil; characteristics and room-odor tests. Journal of the American Oil Chemist Society 67, 161-164.
Przybylski, R., Malcolmson, L.J., Eskin, N.A.M., Durance-Todd, S., Mickle, J. and Carr, R. 1993. Stability of low linolenic acid canola oil to accelerated storage at 60 ºC. Lebensmittel-Wissenschaft und -Technologie. Food Science and Technology 26, 205-209.
Raney, P. and Rakow, G. 1995. A new Brassica napus genotype with superior yellow seed colour and very low alkenyl glucosinolate content. Proceedings of the Ninth International Rapeseed Congress, Cambridge, UK, 4, 1154-1156.
Raney, P., Rakow, G. and Olson, T. 1995. Development of low erucic, low glucosinolate Sinapis alba. Proceedings of the Ninth International Rapeseed Congress, Cambridge, UK, 2, 416-418.
Rashid, A. 1995. An evaluation of seed quality characteristics of near-isogenic yellow and black seeded Brassica napus L. Ph.D. Thesis, University of Saskatchewan, Saskatoon, Canada. 105 pp.
Rimmer, S.R., Scarth, R., McVetty, P.B.E., Woods, D., Gugel, R. and Séguin-Swartz, G. 1995. Breeding for Resistance to Blackleg (Stem Canker) in Western Canada. Blackleg News 5, 11-15.
Siebel, J. and Pauls, K.P. 1989. Inheritance patterns of erucic acid content in populations of Brassica napus microspore-derived spontaneous diploids. Theoretical and Applied Genetics 77, 489-494.
Thies, W. 1971. Schnelle und einfache Analysen der Fettsäurezusammensetzung in einzelnen Raps-kotyledonen I. Gaschromatographische und papierchromatographische Methoden. Zeitschrift für Pflanzenzüchtung 65, 181-202.
Thies, W. 1980. Analysis of glucosinolates via “on-column” desulfation. Proceedings of Symposium “Analytical Chemistry of Rapeseed and its Products” Winnipeg, p. 66-71.