Selection in transgenic lines of oilseed rape (Brassica napus L.) with modified seed oil composition
E. Rudloff 1), H.U. Jürgens 2), B. Ruge1), P.Wehling 1)
Federal Centre for Breeding Research on Cultivated Plants, 1) Institute for Agricultural Crops, 2) Institute for Stress Physiology and Quality of Raw Materials
D-18190 Gross Lüsewitz, Germany
ABSTRACT
Spring-type oilseed rape cv. 'Drakkar' carrying the thioesterase gene ClFatB4 from Cuphea lanceolata which is responsible for the formation of middle-chain fatty acids in the seed oil is used for a field release trial. The expression of ClFatB4 results in the formation of myristic acid (C14:0) as well as an increase of palmitic acid (C16:0) and decreased oleic acid. The correlation between C14:0 and C16:0 is r= +0.8. Both C14:0 and C16:0 are negatively correlated to oleic acid (r= -0.75 and -0.77, respectively). The mean C14:0 contents increased during three cycles of selection from 1.7% - 9.1% in T3 ... 12.9 - 14.1% in T6. In lines displaying a consistently low C14:0 content a single transgene locus was observed by segregation analysis. Transgenic plants did not show striking differences to 'Drakkar' in respect to yield. The oil of the transgenics displays an increase in tocopherol content and oxidation stability. It is solid at +5°C.
Keywords: myristic acid, thioesterase gene, field release
1. Introduction
Oilseed rape (Brassica napus L.) is the most important oilseed crop in Germany. In 1998 it occupied an acreage of about 1 million hectares. Most of the yield is consumed for food. The utilisation for non-food applications, however, is of increasing interest. The industrial consumption of vegetable oil in Germany is about 700,000 metric tons (MT) per year, of which 175,000 MT are contributed by the home market. In addition to the fatty acids (FA) of adapted oilseed crops (oleic acid, erucic acid, linolenic acid) there is considerable demand (400,000 MT per year) of middle-chain fatty acids (MCFA). One these is the myristic acid (C14:0) which is needed for the production of detergents, laundries and cosmetics. Martini et al. (1995) reported on four thioesterase genes with different substrate specificities in Cuphea lanceolata. One of them, ClFatB4, seems to be responsible for the formation of myristic acid (C14:0). In 1996 we started a 10-year field release trial of transgenic spring-type plants carrying this gene (Rudloff et al. 1997). The objects are (1) to study the strength and stability of the gene expression under field conditions, (2) to collect information about the inheritance of the trait, (3) to select lines with high and stable C14:0 content, and (4) to produce sufficient seed lots with high C14:0 content for investigations on the seed and oil processing. The present paper reports on some results of this experiment.
2. Material and Methods
2.1 Material
The released material originated in seven independent original transgenic lines (OTLs) of spring-type oilseed rape cv. 'Drakkar' carrying the thioesterase gene ClFatB4 from Cuphea lanceolata together with its native, seed-specific promotor. The OTLs named T95-1, T95-2, T95-3, T95-4, T95-5, T95-6, and T95-7 were described by Rudloff et al. (1997). The OTLs T95-4 and T95-5 are of generation T3, whereas the other lines are of T2 generation, i.e. the seeds obtained by selfing the primary transgenic plant. Both groups of OTLs were propagated in winter 1995/96 before their release by bagging of single plants in the greenhouse.
2.2 Methods
In an experimental field the lines of all OTLs except of T95-4 and T95-5 are grown to study the genetic and breeding problems including selection. In a separate propagation field the best lines resulting from the selection programme are grown to produce sufficient seed amount for processing studies in a pilot plant scale. In 1996 T95-4 and T95-5 were grown for this purpose. The lines are drilled with a plot seeder HegeÒ or manually seeded in rows, depending on the seed amount. The size of the five-rowed plots is 1.5 x 3.0 m for experimental plots, 1.5 x 6.0 m in yield trials and 1.5 x 12.0 m for propagation, respectively. According to the German regulations on the release of genetically modified organisms the transgenic plots are surrounded by a 6-metre strip of non-transgenic oilseed rape 'Drakkar', which is also used as a check variety. The seed density is about 70 seeds/m2. The seeding date is about mid of April. Field management and data assessment during growth is as usual in breeding. From the experimental lines 15 to 30 individual plants are bagged and harvested separately. Crosses are made by bud pollination with subsequent bagging to avoid selfing or unwanted pollinations. The plants are harvested by hand (manual pollinated inflorescences) or by a Walther&WintersteigerÒ plot harvester, respectively. In each generation seeds of the best plants according chemical analysis are chosen to build up the next generation, i.e. the selection is based on individual plants.
Chemical analyses of seed oil composition are performed by capillary gas chromatography (Rudloff et al. 1997). Two kinds of samples are analysed: (1) a mixture of ten seeds to characterise an offspring, and (2) one seed or a half seed to obtain more detailed information on variability and for half-seed selection. The FAs are abbreviated as follows: myristic acid, C14:0; palmitic acid, C16:0; stearic acid, C18:0; oleic acid, C18:1; linolic acid, C18:2; linolenic acid, C18:3.
Statistical tests were carried out using the SAS /STAT® software version 6 (SAS Institute Inc.).
The investigations on seed and oil processing were undertaken by Pilot Pflanzenöl Magdeburg e.V. (Germany) using industrial standard methods.
3. Results and discussion
3.1 Expression of the transgenic trait
As expected the seed oil composition of the transgenic lines is modified showing C14:0 as a novel trait and an increased content of C16:0. This indicates that the action of the gene ClFatB4 does not only affect the formation of myristic acid, but of palmitic acid, too. The increase of C14:0 and C16:0 has a pronounced effect on C18:1, whereas the content of C18:2 and C18:3 remains nearly constant (fig.1). This is confirmed by the correlation between the main FAs in T6.. There is a close correlation (r= +0.8) between C14:0 and C16:0 and both are closely related to C18:1 with r= -0.75 and -0.77,
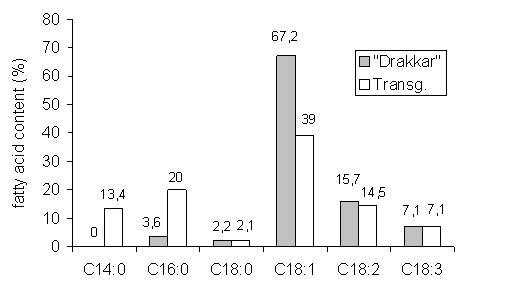 |
Figure 1: Fatty acid composition of transgenic lines in comparison to 'Drakkar'
respectively. The correlation to C18:2 is not significant (r= -0.2 and -0.1) whereas C18:3 is significantly correlated (r= -0.53 and –0.57).
An important criterion of transgene expression is its stability under field conditions. Across three generations we never observed a loss of transgene expression for any of the transgenic lines in the field. The C14:0 contents of T3 seeds produced in the greenhouse in 1996 displayed a strong correlation to T4 seeds harvested in the field with r= +0.71 to + 0.96 for the different OTL. This suggests that under the tested environmental conditions variation of C14:0 expression was mainly genotype dependent with minor, if any, genotype x environment interaction. Thus selection on increased C14:0 content in the greenhouse should be possible, taking into consideration a clear temperature dependence of C14:0 biosynthesis. For instance, 85 randomly chosen half-seed individuals were grown in the greenhouse and compared to 72 half-seed individuals in the field in 1998. The difference of means (13.6% vs. 10.9% C14:0) was significant at a= 0.05 The variation in C14:0 was markedly higher in the greenhouse than in the field (3.9% to 21.0% vs. 3.1% to 15.4%).
3.2 Inheritance of the C14:0 content
In some cases segregation into clearcut classes was observed, suggesting a simple inheritance of the trait. Two selfed progenies of T4 plants expressing relatively low amounts of 1.2% and 1.5%, respectively, as well as the F2 of a cross between 'Drakkar' and a transgenic with 4.2% C14:0 was studied. In all three cases segregation fitted a 1:2:1 ratio indicating a single transgene insertion site with additive action of the transgene copies, each of which contributing approx. 2% C14:0 (table1). Tests of half-seed individuals from T32/11 and T32/20 using ClFatB4-specific primers (Ruge et al. 1998) confirmed absence of the transgene in the 0% class and presence of a specific amplicon in any C14:0-containing individual. The results were verified by use of nptII-specific PCR. The actual number of transgene copies in C14:0-positive plants has yet to be determined by Southern analysis.
Table 1: Segregation of C14:0 content in several progenies of transgenic plants
|
C14:0 content (%) |
0 |
0.1-2.9 |
3.2-5.5 |
c2 1:2:1 |
c2 (a=0.05;2 df) |
||
|
Mean of the class (C14:0%) |
0 |
1.9 |
4.2 |
|
|
||
|
32/11 (T5) |
23 |
40 |
23 |
0.419 |
|
||
|
5.99 |
5 |
17 |
5 |
1.815 |
|
||
|
TF1/54/1 (F2) |
14 |
39 |
10 |
4.079 |
|
||
|
Summarised |
42 |
96 |
38 |
1.636 |
|
3.3 Selection
The lines of all OTLs, except of T95-3, responded to the selection (see chapter 2.2) with an increase of the C14:0 content (tab. 2). The progeny of T95-3 was excluded from further selection because of its insufficient C14:0 level. Particularly from T5 to T6 a striking increase in the mean C14:0 content of the remaining OTLs was observed. This is certainly due to an increased selection intensity in T5 as compared to T4. Obviously, mean C14:0 levels are approaching a plateau under strong selection from T3 through T6. In T6 only T95-6 is significantly different from the other OTLs whereas in T4 only T95-1 and T95-7 were not distinguishable in their means. To increase the variability by recombination crosses were made between lines of the same OTL (type a) and of different OTLs (type b), respectively. As expected, the variability in the F2 is higher in type b crosses (398 plants) than in the type a crosses (346 plants) and the mean C14:0 content is significantly different (10.9% vs. 9.3%). Significant differences also occur between certain crosses. The frequency distribution in C14:0 content of the two types (fig. 2) demonstrates the shift towards higher values in the crosses between OTLs.
Table 2: Selection for C14:0 content in successive selfing generations
(the means are calculated over all lines of the generation)
|
OTL |
|
C14:0 content 1) |
|||
|
|
|
generation |
|||
|
|
|
T3 |
T4 |
T5 |
T6 |
|
T95-1 |
Min. |
5.0 |
5.3 |
3.0 |
11.4 |
|
|
Max. |
11.4 |
11.8 |
12.8 |
16.4 |
|
|
Mean |
7.8 a |
8.8 |
9.8 |
13.0 a |
|
T95-2 |
Min. |
0.1 |
0.0 |
0.0 |
11.5 |
|
|
Max. |
4.5 |
4.1 |
12.0 |
14.9 |
|
|
Mean |
1.7 b |
2.1 |
3.0 |
13.1 a |
|
T95-3 |
Min. |
3.5 |
2.2 |
3.5 |
-2) |
|
|
Max. |
6.3 |
5.8 |
8.1 |
-2) |
|
|
Mean |
4.9 c |
4.6 |
5.4 |
-2) |
|
T95-6 |
Min. |
7.1 |
8.0 |
0.2 |
7.9 |
|
|
Max. |
10.7 |
12.0 |
18.2 |
17.1 |
|
|
Mean |
9.1 d |
10.0 |
10.2 |
14.1 b |
|
T95-7 |
Min. |
5.0 |
5.8 |
3.0 |
4.4 |
|
|
Max. |
10.2 |
11.8 |
14.6 |
18.0 |
|
|
Mean |
7.5 a |
8.6 |
9.7 |
12.9 a |
|
'Drakkar' |
|
|
0.0 |
1.0 3) |
1.3 3) |
1) means followed by the same letter in columns are not significantly different
(Tukey' s studentised range test; a= 0.01)
2) removed from the selection programme in 1997 (see text)
3) open pollinated in the respective yield trial
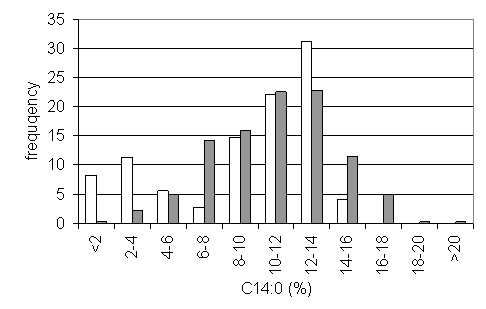 |
Figure 2: The effect of recombination type on the C14:0 content in F2
![]()
![]() ( type a: lines of the same OTL; type b:
lines of different OTLs)
( type a: lines of the same OTL; type b:
lines of different OTLs)
3.4 Seed yield
We observed no disadvantageous phenotypic differences in the growth and development of the transgenics as compared to the check variety 'Drakkar'. In the first year (1996) only few lines had shorter pods, indicating a reduced fertility. This did not occur the subsequent generation, certainly as an effect of selection. Usually the flowering of the transgenics was 4 to 6 days later.
In 1997 and 1998 the best lines of the respective selection were tested in a yield trial. In a block design with three replications 11 lines were compared to 'Drakkar'. The C14:0 content of the tested lines varied from 9.4 to 13.2% in 1997 and 13.2 to 13.4% in 1998. In 1997 two of 11 transgenic lines displayed significant lower yields than 'Drakkar', whereas in 1998 no significant differences were found.
3.5 Processing of seed and oil
In 1996 seed lots of 30 kg each of T95-4 and T95-5 as well as 5 kg of a mixture of the best lines were chosen for a comparison with 'Drakkar'. The processing was carried out in a pilot plant scale. The rapeseed was pressed, extracted with hexane and refined. In every step of processing the fatty acid composition and other important components of the oil were analysed. In the processing of seed and oil no striking differences to other oilseed rape were observed. Some parameters of oil qualityare given in table 3. Oil of transgenics is solid at +5°C even in lines with relatively low C14:0 contents.
Table 3: Oil properties of transgenes in comparison to 'Drakkar'
|
|
'Drakkar' |
T95-4 |
T95-5 |
Mixture |
|
Oil (%)1) |
39.1 |
41.3 |
43.7 |
39.9 |
|
C14:0 (%)1) |
0.1 |
1.9 |
4.2 |
9.1 |
|
Tocopherol (mg/kg) 2) |
242.7 |
274.2 |
303.8 |
344.3 |
|
Oxidation stability at 100°C (hours) 2) |
16.5 |
21.8 |
22.7 |
29.5 |
1) analysed in the seed
2) analysed in the refined oil
4. Conclusions
The level and stability of C14:0 expression as well as the behaviour of transgenes in the field indicate a stable integration of the ClFatB4 gene in the 'Drakkar' genome without disadvantages for growth and yield. The individual selection on increased C14:0 content was successful and may be further enhanced by recombination via crosses between different OTLs. The oil of the transgenics displays changes in chemical and physical properties, e.g. low amount of unsaturated FA, higher tocopherol content and higher oxidation stability, which may be interesting for industrial use.
Acknowledgements
We thank Mr. N. Martini from the "Max-Planck-Institut für Züchtungsforschung Köln-Vogelsang" for kindly providing of the transgenic lines as well as the "Union zur Förderung von Öl- und Proteinpflanzen e.V. Bonn" (Germany) for financial support of the investigations on seed and oil processing.
References
Martini N., Schell J. and Töpfer R., 1995. Expression of medium-chain acyl-(ACP) thioesterases in transgenic rapeseed, in: Rapeseed Today and Tomorrow. Proc. 9th Intl. Rapeseed Congress, 4-7 July 1995 Cambridge, UK., vol. 2: pp. 461-463.
Rudloff E. and Wehling P., 1997. Release of transgenic oilseed rape (Brassica napus L.) with altered fatty acids, in: Thomas, G. and Mionteiro, A.A. (eds.) Proc. Intl. Symp. on Brassicas, Rennes (France) 22-27 September 1997, Acta Hort. 459, pp. 379-385
Ruge B., Rudloff E., Sonntag K. and Wehling P., 1998. Entwicklung molekularer Nachweisverfahren für Transgene im Raps, Vortr. Pflanzenzüchtg., Heft 43, S. 194-201