ANTISENSE RNA TO DECREASE THE GREEN SEED PROBLEM IN
CANOLA
Anne M. Johnson-Flanagan, Nancy Go, Fengcheng Sun, Jas Singh*, Laurian
Robert* and Michele N. Konschuh,
Department of Agricultural, Food & Nutritional Science, Faculty of Agriculture, Forestry and Home
Economics, University of Alberta, Edmonton, Alberta, Canada, T6G 2P5, e-mail aflanaga@gpu.srv.ualberta.ca and
*Eastern Cereal and Oilseed Research Centre, AAFC, Ottawa, Canada.
ABSTRACT
The objective of this research was to determine whether chlorophyll in green canola seed could be
decreased under normal seed maturation conditions and following frost, through the use of antisense
RNA, without affecting the quality and quantity of storage protein and oil. We intended to limit the level
of chlorophyll through the production of the antisense form of Type I CAB of photosystem II in
transgenic Brassica napus, cv Westar seeds. We used the seed specific promoter for napin, which
directs a high level of gene transcription.
The results from the T5 generation indicate that the amount of chlorophyll a and b in the seed was
significantly decreased under control conditions. The reduction was correlated with suppression of both
CAB transcripts and protein. Following exposure to frost, the seed often undergoes renewed pigment
synthesis. This increase was again suppressed in the transgenics. The combination of decreased
accumulation under control conditions and decreased synthesis following frost led to low levels of
pigment in the seed shortly after the frost. More importantly, mature seed from the transgenics
contained little or no pigment, whereas the wild type controls were green.
KEYWORDS chlorophyll, freezing stress, chlorophyll a/b binding proteins
INTRODUCTION
Canola, an important oilseed crop in Canada, is graded on a number of parameters including the
quantity of protein, quantity and quality of lipids and the percent of green seed. As little as 6% green
seed results in downgrading of the crop. Pigments in green seed are extracted into the oil, imparting
off-flavors and odors and reducing the shelf-life of the oil. The current method of removing pigment
from canola oil involves the use of bleaching clays which are costly, cannot be re-used, and must be
sent to landfill sites. Therefore, alleviating green seed in canola is an industry priority.
One cause of green seed is exposure of canola to sub-lethal frost (0 to 5C) during seed development
(Johnson-Flanagan et al., 1990b). Sub-lethal frost has been shown to induce renewed pigment
synthesis and rapid desiccation in seed (Johnson-Flanagan et al., 1990b; 1991).
In leaves, there is a known correlation between the amount of chlorophyll and specific chlorophyll a/b
binding (Cab) proteins. Cab proteins degrade in the absence of chlorophyll (Cuming and Bennett,
1981), and similarly, chlorophyll accumulation appears to be dependent upon appropriate folding of Cab
proteins (Plumley and Schmidt, 1995). It has also been demonstrated that a similar relationship exists
between Cab proteins and chlorophyll in green haploid embryos and seed of Brassica napus (Politeski-
Morissette et al., 1998). This co-dependent relationship between Cab proteins and chlorophyll formed
the basis for plant transformations with an antisense cab gene. It was hypothesized that a reduction in
Cab protein accumulation in seed of Brassica napus should bring about a reduction in chlorophyll
accumulation in canola seed.
MATERIALS AND METHODS
Plant material
Flowers were hand pollinated and tagged at anthesis in order to stage development. T6 seed was
collected from homozygous T5 plants of two independent transformants, DII and DIII. Comparisons
were made with wild type Westar plants.
Frost treatment
Westar, DII and DIII plants were divided into two groups; frozen and not frozen. Intact plants at 26, 30
and 34 DPA were placed in a programmable freezer at 0C. Following a 1 h equilibration period, the
temperature was lowered by 5 C h-1 to -5C, followed by a 3 h equilibration period. Then the
temperature was raised by 5C h-1, plants were removed at 0C, allowed to thaw at 4C overnight and
then returned to the greenhouse. Seed was collected from frozen and non-frozen plants 1 and 4 days
after treatment and at maturity. Samples were pooled, frozen in liquid nitrogen, then used for RNA,
protein and chlorophyll extractions. The entire experiment was repeated three times.
RNA extraction and northern blotting
Blots of total RNA were hybridized with a 32P-labelled riboprobe (32P-UTP and RNA Labelling Kit,
Amersham) from the antisense orientation of the cab gene.
Immunoblotting
Total SDS-soluble polypeptides were separated by SDS-PAGE and transferred to membranes. The
primary antibody was -CP1a. The membranes were processed with an alkaline phosphatase
secondary antibody and Fast Red stain according to White and Green (1987).
Chlorophyll determination
Chlorophyll from 20 to 30 seed was extracted in 80% acetone using the method of Vernon (1960).
Absorbance of three aliquots from each extract was read at 649 and 665 nm against an 80% acetone
blank in a Beckman spectrophotometer. Total chlorophyll, chlorophyll a and chlorophyll b were
calculated using the equations of Vernon (1960).
RESULTS AND DISCUSSION
Our previous work shows that seed chlorophyll content increases until approximately 28 DPA or 55%
seed moisture and decreases thereafter (Johnson-Flanagan and Thiagarajah, 1990a; Politeski-
Morissette, 1998). These changes are paralleled by changes in cab gene expression (Politeski-
Morissette et al., 1998). We have also shown that exposure to sublethal frost at this stage of
development results in the greatest retention of pigment in the mature seed (Johnson-Flanagan et al.,
1990b). Therefore, in the present study, we examined cab gene expression, cab protein levels and
chlorophyll content in seed from plants that had been frozen between 26 and 34 DPA. Exposure of
Westar to sub-lethal frost resulted in an increase in sense cab RNA levels 1 day after treatment relative
to levels in seed from non-frozen plants (Fig. 1). In contrast, levels of sense cab RNA in the transgenic
lines increased marginally (DIII at 26 DAP), remained unchanged (DII and DIII at 30DP) or decreased
substantially. These differences were maintained at 4 days after treatment, with the exception of the 34
DPA samples. At this developmental stage, cab expression was quite low and the differences between
the transgenics and wild type was minimal. The data for 30 DAP are shown in Figure 1a and b.
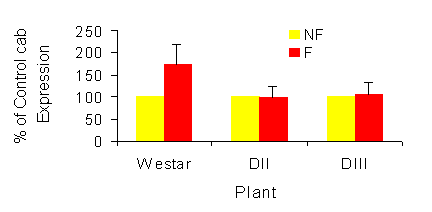 |
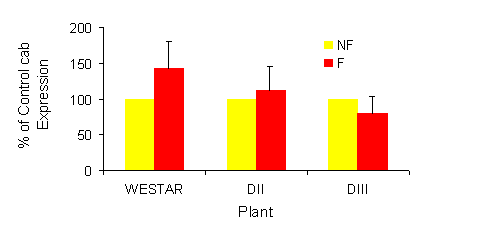 |
Fig. 1a and b. Effect of sub-lethal frost on expression of sense cab RNA in Brassica napus
seed. Non-frozen controls are normalized to 100%. Values represent the means + SE, n=3. a,
one day after frost; b, four days after frost. NF = Non frozen, F = Frozen.
Differences in Cab protein accumulation have been shown to correlate with chlorophyll content
(Politeski-Morissette et al., 1998). Exposure to sub-lethal frost resulted in an increase in Cab protein
accumulation in seed of all the plants. However, the increase was less in the transgenic plants. For
example, plant exposed to frost at 30 DPA and assayed 4 days later showed a large increase in Cab in
Westar and smaller increases in the transgenic lines (Fig. 2).
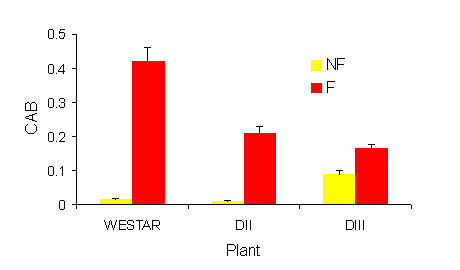 |
Fig. 2. Effect of sub-lethal frost on Cab protein accumulation in Brassica napus seed 4 days
after frost. Values represent the means + SE, n=3. NF = Non frozen, F = Frozen.
In seed from non-frozen plants, the transgenics contained less chlorophyll in comparison to Westar.
Following a sub-lethal frost, chlorophyll remained at essentially the same level as in the non-frozen
controls for 1 day (Fig 3a) and then decreased, retaining the same order in terms of quantity of
chlorophyll as was seen in the non-frozen controls. While these results were promising, it was the
results from the mature seed that clearly indicated that the technology worked (Fig. 3b). In the mature
seed, Westar plants that had been exposed to frost had a very significant green seed problem. On the
other hand, seed from the transgenic lines had no more chlorophyll than the non-frozen counterparts.
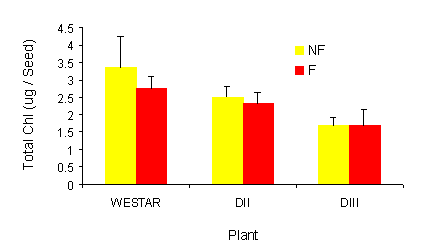 |
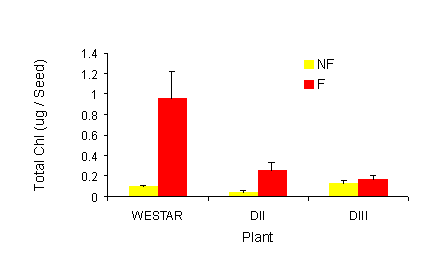 |
Fig. 3 a and b. Effect of sub-lethal frost on seed chlorophyll content. Total chlorophyll
(g/seed) was extracted from frozen and non-frozen seed (30 DPA). Values represent the means
+ SE, n=3. a, one days after frost; b, at maturity. NF = Non frozen, F = Frozen.
Our previous results demonstrated that antisense cab could reduce the accumulation of chlorophyll in
developing canola seed (Politeski-Morissette et al., 1998). While this was an important finding, green
seed is usually the result of stress, most notably frost. The results from the present study indicate that
retention of chlorophyll in the seed following exposure of canola plants to sublethal frost is significantly
reduced by expression of antisense cab.
REFERENCES
Cuming A. and Bennett J. 1981. Biosynthesis of the light-harvesting chlorophyll a/b protein. Eur. J.
Biochem. 118: 71-80.
Johnson-Flanagan A. and Thiagarajah M. 1990a. Degreening in canola (Brassica napus cv. Westar)
embryos under optimum conditions. J. Plant Physiol. 136: 180-186.
Johnson-Flanagan A., Singh J. and Thiagarajah M. 1990b. The impact of sublethal freezing during
maturation of pigment content in seeds of Brassica napus. J. Plant Physiol. 136: 385-390.
Johnson-Flanagan A., Thiagarajah M. and Pomeroy K. 1991. The impact of freezing during maturation
on storage products in canola seeds. Physiol. Plant. 81: 301-308.
Plumley G. and Schmidt G. 1995. Light-harvesting chlorophyll a/b complexes: Interdependent pigment
synthesis and protein assembly. The Plant Cell. 7: 689-704.
Politeski Morissette J.C., Konschuh M.N., Singh J., Robert L. and Johnson-Flanagan A.M. 19978.
Reduction of chlorophyll accumulation in seed of tranasgenic Brassica napus using antisense
technology. Acta Horticulturae 459:183-190.
Vernon L. 1969. Spectrophotometric determination of chlorophylls and phaeophytins in plant extracts.
Anal. Chem. 32: 1144-1150.
White M.J. and
Green B.R. 1987. Antibodies to the photosystem I chlorophyll a + b antenna cross-react with
polypeptides of CP29 and LHCII. Eur. J. Biochem. 163: 545-551.
ACKNOWLEDGEMENTS
Funding was provided by AARI, Western Grains Research Foundation, NSERC-AAFC and Pioneer Hi-
Bred.