ROLE OF RHIZOCTONIA SPECIES IN DAMPING-OFF OF CANOLA
ABSTRACT
Hypocotyl rot and damping-off have been associated with cases of poor stand establishment or complete stand failure of canola seedlings in Western Australia since 1994. Isolations indicated that Rhizoctonia spp. were associated with these conditions. A total of 127 Rhizoctonia isolates were collected from various canola growing areas during 1994-98. Pectic enzyme electrophoresis differentiated these isolates into six distinct zymogram groups; R. solani, ZG5 (AG2-1), ZG6 (AG2-1), ZG9 (AG10); binucleate Rhizoctonia, CZG1, CZG4, CZG5 (AGK); and unidentified binucleate groups. Binucleate groups were confirmed by fluorescent nuclear staining and hyphal morphology. One or more isolates from each of the above zymogram groups (including four unidentified binucleate groups) and an isolate of ZG1-1 (AG8), that causes bare patch in cereals and legumes, were tested for their pathogenicity on canola. Isolates of ZG5 and ZG1-1 were highly pathogenic on canola, delayed seedling emergence and caused severe hypocotyl or root rot, respectively. ZG5 also induced post emergence damping-off. Increasing the depth of sowing from 1 to 3 cm significantly delayed seedling emergence and increased disease severity. Four unidentified binucleate isolates were moderately pathogenic to canola, while two isolates caused significant pre-emergence damping-off. Two binucleate isolates of CZG5 were weakly pathogenic. Efficacy of various seed dressing fungicides to control hypocotyl rot/damping-off caused by ZG5 is also discussed.
KEY WORDS: Brassica. napus, hypocotyl rot, zymogram , pathogenicity, electrophoresis.
Rhizoctonia solani Kuhn reduces both emergence (pre-emergence damping-off) and stand density (post-emergence damping-off) in crucifers and other crops (Sweetingham, 1996). Hypocotyl rot has been associated with some cases of poor stand establishment or complete stand failure of canola in Western Australia since 1994 (Khangura and Barbetti, 1996). Isolations showed that Rhizoctonia spp. are associated with hypocotyl rot and damping-off of canola in WA (Khangura and Barbetti, 1996). The objectives of this study were to characterise the zymogram groups of R. solani and test their pathogenicity on canola and to evaluate various seed dressing fungicides to control hypocotyl rot in canola.
MATERIALS AND METHODS
Collection of Rhizoctonia isolates and their pathogenicity on canola
Canola seedlings showing symptoms of hypocotyl rot or damping-off were collected from various canola growing regions during 1995-98. After surface sterilisation, diseased portions were cut into small segments and plated on 2% water agar. After 48 hours colonies resembling Rhizoctonia were subcultured on potato dextrose agar containing aureomycin hydrochloride. The identity of these isolates was confirmed based on hyphal morphology and zymogram groups were determined using electrophoresis (Sweetingham et al, 1986). The pathogenicity of isolates of each zymogram group and one isolate of ZG1-1 group (causing bare patch in cereals) was tested on canola in the glasshouse. Four isolates of Rhizoctonia (WAC9307, WAC9316, WAC9297 and WAC9290) each representing an unidentified binucleate group and two isolates of CZG5 (WAC9308 and WAC9324) isolated from diseased canola seedlings were tested for their pathogenicity on canola cv. Narendra. . Plastic pots (9 cm diameter) of 1 kg capacity were half filled with soil mixture (80% river sand: 20% yellow sand), then 6 millet seeds colonized by the respective Rhizoctonia isolate were placed on the soil surface and the pot filled with soil mixture. Pots with sterile uninoculated millet seeds were included as controls. Pots were maintained in a phytotron cabinet with day/night temperature of 18/15°C, 12h photoperiod and light intensity of 300 mE/m2/s. After 2 weeks, 12 surface sterilized canola (B. napus L.) seeds cv Oscar. For all the pathogenicity experiments the plants were rated on a 0-3 scale for the hypocotyl rot and 0-5 scale for the lateral and tap root rot. Percent disease index was calculated as: sum of disease ratings / (number of plants in all categories x maximum grade on a rating scale) x 100 (where sum of disease ratings = no of plants in a disease category x numerical value of a disease category).
Efficacy of various seed dressing fungicides to control hypocotyl rot
In a field-plot trial efficacy of 7 different seed dressing fungicides was tested to control hypocotyl rot caused by ZG5. The experimental area was inoculated with ZG5 inoculated millet seeds at the rate of 1000 millet seeds/m2 and incorporated to a depth of 5 cm with a rotary hoe. The fungicides (Apron FDL, BenlateÒ, Fluquinconazole, RovralÒ, ThirafloÒ, Triticonazole and VitavaxÒ) were used in these studies and each fungicide was applied to the seed @ 4g a.i/Kg of the seed. Observations on initial seedling emergence were made 3 weeks after sowing and the healthy plants were counted 4 weeks later.
RESULTS AND DISCUSSION
Collection of Rhizoctonia isolates and their pathogenicity on canola
A total of 127 isolates were obtained from diseased canola seedlings during 1995-1998. Pectic zymogram investigations of these isolates revealed that of the isolates belonged to ZG5 group, 8% to ZG6, 7% to CZG1, and 3% each to CZG 4 and CZG5 respectively. Remaining isolates were grouped under unidentified binucleate groups. Pathogenicity studies showed that strain ZG5 was the most pathogenic on canola and caused up to 70 % post-emergence damping off in controlled experiments. This strain caused hypocotyl rot on the surviving canola seedlings while strain ZG1-1 caused severe tap root rot (43%) on canola (Fig. 1). These two strains also caused lateral root rots (17% and 19%) respectively. In contrast, the other strains tested, CZG1, CZG4 and CZG5 (attacking cereals and legumes) and ZG6 (causing root and hypocotyl rot in legumes), were only weakly pathogenic on canola (Fig. 1). In case of pathogenicity with unidentified binucleate isolates, WAC9316 produced the highest disease index of hypocotyl rot (44%) followed by isolates WAC9297, WAC9307 and WAC9290 with the disease indices of 40, 39 and 38% respectively, these differences were not significant. Isolates WAC9324 and WAC9308 were only weakly pathogenic with low disease indices of 5% and 7% respectively (Fig. 2).
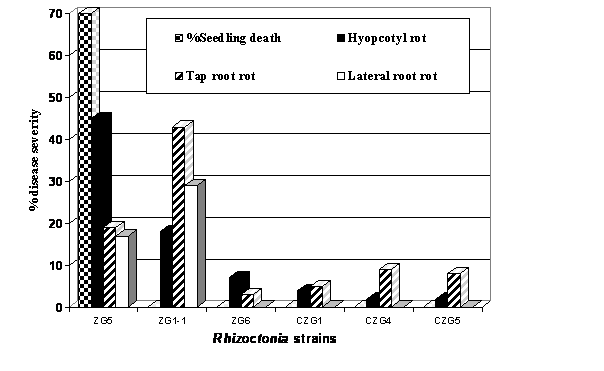
Fig. 1 Pathogenicity of identified Rhizoctonia
isolates on canola

Fig. 2 Pathogenicity of unidentified binucleate Rhizoctonia isolates on canola
Efficacy of various seed dressing fungicides to control hypocotyl rot
Initial seedling emergence varied greatly among different treatments (20-65%) and was substantially improved with Rovral followed by Fluquinconazole compared with the other fungicides and the control. However, the percent post-emergence damping-off was substantially reduced with different fungicide treatments. Fluquinconazole was the best fungicide treatment to reduce percent damping-off (less than 10%) compared to other treatments and control.
Conclusions
In conclusion, ZG5 is the most predominant, highly pathogenic strain and causes hypocotyl rot and post-emergence damping-off of canola. In contrast, ZG1-1, which causes bare-patch in cereals and legumes, can also cause serious root rot in canola. The known binucleate strains generally are less pathogenic on canola. Some unidentified binucleate types present in some situations could also cause hypocotyl rot on canola. Seed dressing fungicides such as Fluquinconazole and RovralÒ can be used to control hypocotyl rot caused by ZG5 in canola.
ACKNOWLEDGMENTS
We thank Grains Research and Development Corporation for providing funding for this research. We also thank Cheryl Wallace and Robert Davidson for technical assistance.
REFERENCES
Khangura, Ravjit and Barbetti, M.J. (1996). Management of fungal diseases of canola for sustainable rotations in Western Australia. Western Australian Oilseeds Update Meeting for Advisers and Consultants, Feb 1996. pp 28-30.
Sweetingham, M.W., Cruickshank, R.H. and Wong, D.H. (1986). Pectic zymograms and taxonomy and pathogenicity of the Ceratobasidiaceae. Transactions of the British Mycological Society 86, 305-11.
Sweetingham, M.W. (1996). Integrated control of Rhizoctonia species. In ‘Rhizoctonia species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control,’ eds. B. Sneh, Jabaji-Hare, S., Neate, S. and Dijst, G. pp. 549-58. (Kluwer Academic Publishers, Netherlands).