EUROPEAN PESTS OF RAPESEED: A THREAT TO AUSTRALIAN CROPS?
J.M. Hughes and K.A. Evans
SAC, West Mains Road, Edinburgh, EH9 3JG, United Kingdom
ABSTRACT
The climatic response of two European oilseed rape pests, Dasineura brassicae Winn. and Ceutorhynchus assimilis Payk., was experimentally investigated and integrated with literature reports of each species’ distribution, abundance and seasonal phenology using CLIMEX, a computer-based simulation system. Australian climatic suitability and the influence of indigenous biotic factors were estimated, and the overall survival and damage potential of introduced pest populations predicted.
It is predicted that the main oilseed cultivation areas of Australia would support economically damaging populations of C. assimilis and the C. assimilis/D. brassicae pest complex. However, D. brassicae is largely dependant upon C. assimilis damage for oviposition and although alternative forms of pod damage would allow population establishment, there is no native pod-damaging mechanism which would allow economically damaging population levels to be achieved were D. brassicae to be introduced without C. assimilis.
KEYWORDS; Dasineura brassicae, Ceutorhynchus assimilis, CLIMEX, Quarantine
INTRODUCTION
European oilseed rape crops are host to a variety of insect pests, however those species which damage the reproductive parts of the plant, such as the cabbage seed weevil (Ceutorhynchus assimilis), the brassica pod midge (Dasineura brassicae) and the pollen beetle (Meligethes aeneus F.) are the most damaging. Both Brassica napus L. (swede rape) and B. campestris L. (turnip rape) are of Mediterranean and Asian origin (Weiss, 1983) and the insects most harmful to oilseed crops also originate from Europe and Asia (Bonnemaison, 1964). In other continents, such as Australasia, rape pests are generally indigenous polyphagous species which are predominately vegetation attacking and are rarely economically damaging to the crop (Bonnemaison, 1964).
Increased intercontinental travel and trade has improved the opportunities available to insects to overcome geographic restrictions of movement from their area of origin. The introduction and establishment of European oilseed rape pests in Australia would have serious implications for the country’s expanding rapeseed production industry. The research described herein estimates the Australian damage potential of introduced populations of two important insect pests of European rape crops; the brassica pod midge (Dasineura brassicae Winn.) and the cabbage seed weevil (Ceutorhynchus assimilis Payk.).
METHODS
It is generally accepted that climate is most often the principal factor limiting the geographic distribution of insects within their native range (Andrewartha & Birch, 1954; Edwards & Heath, 1964). CLIMEX, a computer based simulation system, integrates meteorological information and species-specific parameters to produce a single index, the Ecoclimatic Index, describing the climatic favourability of a location for the development and persistence of an ectothermic population.
The rationale underlying CLIMEX is the assumption that the distribution of an ectothermic species is dominantly determined by climate. The program is designed to forgo the need for in-depth experimental investigation of a species’ climatic response in order to make predictions of potential range, and yet provide a more sophisticated tool than simple climate matching. The authors of the program suggest that all initial parameters should be fitted by inference from reports of distribution, abundance and seasonal phenology throughout the range, and then fine-tuned by experimental data available from the literature (Skarratt et al., 1995). Because of the inherent difficulties in attributing patterns of distribution and abundance to climatic variables without being able to gauge the contributory influence of other factors, and the lack of experimental data in the literature, each species’ mortality and developmental duration was investigated in response to both temperature and soil moisture in a series of experiments designed to complement the parameters used in CLIMEX. The data obtained from the experiments were not treated as absolute values but as a guide to corroborate, and to gain an overview to consolidate, the knowledge gained by inference from the species’ native range, abundance and seasonal phenology. The final climatic profiles constructed in CLIMEX were used to predict the climatic favourability of Australian locations for population survival and increase.
The climatically-controlled population density of an introduced population will be further modified by the local habitat, availability and quality of host plants, and the action of natural enemies and competitors. Therefore the role of such factors were also considered. In addition, D. brassicae has a weak ovipositor, which morphological studies have shown is not adapted for boring through the pod surface (Stechmann & Schütte, 1978; Hallberg & Åhman, 1987) and egg-laying is largely confined to pre-damaged pods. It is generally accepted that D. brassicae primarily relies on C. assimilis damage to provide oviposition access (Ankersmit, 1956; Stechmann & Schütte, 1978; Ferguson et al., 1995). Therefore, the absence of a suitable pod access mechanism may preclude or limit D. brassicae population persistence. Whilst there is no Australian pod damaging species which is solely associated with oilseed rape, one possible alternative vector of D. brassicae oviposition is the Rutherglen bug (Nysius vinitor Berg, Heteroptera: Lygaeidae). N. vinitor is reported to be a pest of Australian rape crops (Matheson, 1976) and feeds by piercing the vegetation and pods of the host, leaving a necrotic lesion at the point of entry (Miller, 1956). Experiments were conducted to investigate the efficacy of damage by a similar UK Lygid spp. (Lygus pabulinus) as a pod access mechanism for D. brassicae.
RESULTS
Within Australia, positive EI values are mainly displayed in the south east of the country, in Tasmania, Victoria and New South Wales (Figure 1). Predicted climatic suitability is greatest in coastal regions, with EI values of up to 100 indicating that the climate is within optimal limits all year round. Locations in coastal Queensland, coastal south Australia and the south west coast of Western Australia are also indicated to be climatically suitable for population persistence of both species. Inland areas and north of the country are predicted to be unsuitable for population survival due to lethal heat and dry stress accumulation.
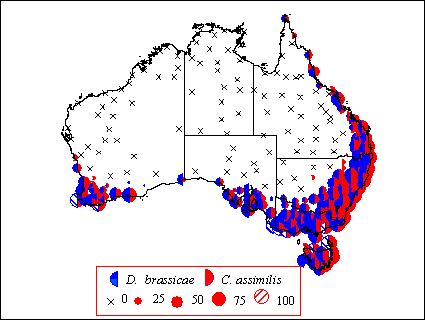
Figure 1 Predicted Ecoclimatic Indices (EI) generated for C. assimilis and D. brassicae at 228 Australian locations. The magnitude of the EI reflects the climatic suitability of the location.
Oilseed rape cultivation is increasing in Australia, the area of rape cultivated quadrupled from 107,109 ha in 1992 to 421,000 ha in 1996 (FAOSTAT Database). Australian oilseed cultivation takes place in the medium-high rainfall areas of the wheatbelt and inland irrigated areas of southern Australia, in an arc from central NSW through central and south-western Victoria, southern South Australia and the wheatbelt of Western Australia (Matheson, 1976). Therefore, the main cultivation areas coincide with those areas where the climate is predicted to be suitable for both C. assimilis and D. brassicae population establishment providing plentiful hosts to allow population establishment and increase.
In addition to climatic suitability throughout the main rape production areas, the freeing from indigenous natural enemies that is conferred by a move to a new continent may result in higher population levels occurring than in the native range. Within their indigenous European range, both C. assimilis and D. brassicae are attacked by a variety of hymenopteran parasites and high levels of parasitism are often recorded. Although polyphagous species may accept the newly introduced species as an alternative host, they are less likely to be as specialised, and therefore as efficient, as the predators present in the indigenous range (Price, 1987).
The experimentation performed to elucidate the efficacy of lygid feeding lesions as a pod access mechanism found that, where no choice is given, there is no difference in oviposition rate between lygid and C. assimilis damage. However, the timing of lygid presence on rape crops is likely to decrease its suitability as a pod access vector. Whilst the seasonal phenology of C. assimilis ensures that there is adequate pod access throughout the rape growing season, lygid bugs characteristically colonise different host plants in each generation and migrate from crop to crop as the season progresses, not colonising rape until mid-summer (Kelton, 1975; Cleveland, 1982). The first and second generations of D. brassicae are the largest, with increasing numbers entering diapause after each generation. Because of their phenology, lygids would not be present to provide oviposition access to the first generation of D. brassicae. Although D. brassicae may oviposit on young undamaged pods and those damaged by wind, disease and other phytophagous organisms, without an abundant and temporally suitable damage provider such as C. assimilis, populations are extremely unlikely to reach economically damaging proportions.
In conclusion, the combination of favourable climatic conditions, an increasingly abundant source of host plants, and an absence of indigenous natural enemies would suggest that both C. assimilis and the C. assimilis/D. brassicae complex would persist and increase to economically damaging levels on Australian oilseed rape crops were they introduced. However, due to a lack of a suitable pod access mechanism, D. brassicae is unlikely to be an important pest of Australian rape crops if it were to introduced without C. assimilis.
DISCUSSION
It is not possible to exactly quantify the presence and relative importance of the survival and abundance influencing factors in a new ecosystem, nor how a population will react to them. There are many factors which cannot be predicted and which may differentially influence populations in areas for which predictions have been made. For example, the phenology of the species in the country of introduction may not be the same as in the country of origin, especially when the former offers a year round climatic optimum. Although in both species diapause is obligate, the phenology in the new area is very much dependant upon the timing of introduction, and the developmental stage of the insect at that time. Whilst univoltine species such as C. assimilis are less likely to deviate greatly from their native phenology, the response of the multi-voltine D. brassicae is more difficult to predict. D. brassicae diapause is thought to be initiated by a combination of temperature and solar radiation (Axelsen et al., 1997) and the timing of attainment of the initiating factors may vary according to continent. In addition, D. brassicae can remain in diapause for up to five years (Ankersmit, 1956; Williams et al., 1987a). If the population were to be introduced whilst in diapause it is impossible to predict the timing of emergence and how it will coincide with the growing season. In addition, the effect of high temperatures on diapausing insects has not been investigated, and it is possible that mortality may be greater when temperatures are above the developmental threshold throughout the year, thus reducing population growth.
However, the investigations implemented herein allow an estimate of colonisation potential to be made by considering the probable influence of the main abundance modifying factors in the area of introduction and indicate that both C. assimilis and D. brassicae would pose a serious threat to Australian oilseed rape production were they to be introduced, raising quarantine implications for these species which can also be extrapolated to other European pests.
REFERENCES
Andrewartha, H.G. & Birch, L.C. (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Ankersmit, G.W. (1956) The biology and control of the colza seedpod weevil (Ceutorhynchus assimilis Payk.) and the brassica pod midge (Dasyneura brassicae Winn.). Insitut voor Plantziektenkundig Onderzoek Wageningen Mededelingen 132: 1-59
Axelsen, J.A., Fink, R. & Kjaer, C (1997) Global solar radiation as the factor controlling induction of diapause in the pod midge (Dasyneura brassicae Winn.). Oecologia 111: 178-182
Bonnemaison, L. (1964) Insect pests of crucifers and their control. Annual Review of Entomology 10: 233-256
Cleveland, T.C. (1982) Hibernation and host plant sequence studies of tarnished plant bugs, Lygus lineolaris, in the Mississippi delta. Environmental Entomology 11: 1049-1052
Edwards, G.A. & Heath, G.W. (1964) The principles of agricultural entomology. Chapman & Hall, London.
FAOSTAT Database - Food and Agriculture Organisation of the United Nations Statistics Database (http://apps.fao.org/default.htm)
Ferguson, A.W., Kenward, M.G., Williams, I.H., Clark, S.J., Kelm, M. & Dudzic, A. (1995) Interactions between the cabbage seed weevil (Ceutorhynchus assimilis Payk.) and the brassica pod midge (Dasineura brassicae Winn.) infesting oilseed rape pods. Proceedings 9th International Rapeseed Congress, Cambridge 4-7th July 2: 679-681
Hallberg, E. & Åhman, I. (1987) Sensillar types of the ovipositor of Dasineura brassicae: structure and relation to oviposition behaviour. Physiological Entomology 12: 51-58
Kelton, L.A. (1975) The lygus bugs (genus lygus Hahn) of North America (Heteroptera: Miridae). Memoirs of the Entomological Society of Canada No. 95: 5-65
Matheson, E.M. (1976) Vegetable oilseed crops in Australia. Holt, Rinehart & Winston, Sydney pp: 82-96
Miller, N.C.E. (1956) The biology of the heteroptera. Leonard Hill Books Ltd., London
Price, P.W. (1987) The role of natural enemies in insect populations. In: Barbosa, P.A. & Schultz, J.C. (Editors) Insect outbreaks. Academic Press, San Diego pp: 287-312
Skarratt, D.B., Sutherst, R.W. & Maywald, G.F. (1995) User’s guide: CLIMEX for Windows version 1.0. CSIRO/CTPM, Brisbane
Stechman, D.H. & Schütte, F. (1978) Endophytic oviposition by Dasineura brassicae Winn. (Dipt., Cecidomyiidae). Zeitschrift Angewandte Entomologie 85: 412-424
Weiss, E.A. (1983) Oilseed crops. Longman Inc., New York
Williams, I.H., Martin, A.P. & Kelm, M. (1987) The phenology of the emergence of brassica pod midge (Dasineura brassicae Winn.) and its infestation of winter oilseed rape (Brassica napus L.). Journal of Agricultural Science, Cambridge 108: 579-589