Syntheses in 4-hydroxyglucobrassicin series : preparation of 4-benzyloxydesulfoglucobrassicin
Christian Gardrat, Karine Darriet and Bernard de Jéso
Laboratoire de Chimie des Substances Végétales, 351 Cours de la Libération F-33405 Talence Cédex France
e-mail : c.gardrat@ipin.u-bordeaux.fr
ABSTRACT
The preparation of 4-benzyloxydesulfoglucobrassicin, a key intermediate for the synthesis of the naturally occurring indole glucosinolate 4-hydroxygluco-
brassicin, is described for the first time.
KEYWORDS : indole glucosinolates, 2-methyl-3-nitrophenol, 4-benzyloxyindole, 4-benzyloxy-3-(2-nitrovinyl)indole
INTRODUCTION
Glucosinolates constitute an important class of compounds widely distributed in all crucifer plants (Tookey et al., 1980 ; Van Etten et al., 1983 ; Fenwick et al., 1983). Much work has been devoted to rape. The meal remaining after oil extraction is fed to livestock, but in limited amounts for glucosinolates are degraded by an endogenous enzyme, myrosinase (E.C. 3.2.3.1) to form in particular, isothiocyanates and thiocyanate ion, nitriles and oxazolidinethiones (Tookey et al., 1980 ; Van Etten et al., 1983 ; Fenwick et al., 1983). These species have been found to be harmful when consumed by humans and animals and can, for example, cause thyroid, liver and kidney diseases in monogastric animals (Tookey et al., 1980 ; Van Etten et al., 1983 ; Fenwick et al., 1983 ; Vermorel et al., 1987, 1988).
New varieties containing less glucosinolates (00 rapeseed) are nowadays cultivated. This genetic modification has affected the content of the various glucosinolates in seeds and indole glucosinolates generally predominate (Slominski and Campbell, 1987). This glucosinolate subclass is very interesting because of its potential anticarcinogenic properties (Mc Dannell et al., 1988 ; Nugon-Baudon and Rabot, 1994). For example, enzymatic degradation products of glucobrassicin inhibit carcinogenesis in rodents and present a powerful activity against hormone-dependent cancers (Huang et al.,1994 ; Telang et al., 1997).The syntheses of several indole glucosinolates have already been described (Viaud and Rollin, 1990 ; Viaud et al., 1992). It is assumed that the presence of an hydroxyl group on an indole moiety should bring antiradical properties which putatively may increase anticarcinogenic activities. In this paper we described the preparation of 4-benzyloxydesulfogluco-brassicin, a key intermediate for the synthesis of natural 4-hydroxyglucobrassicin.
preparation of 4-benzyloxydesulfoglucobrassicin
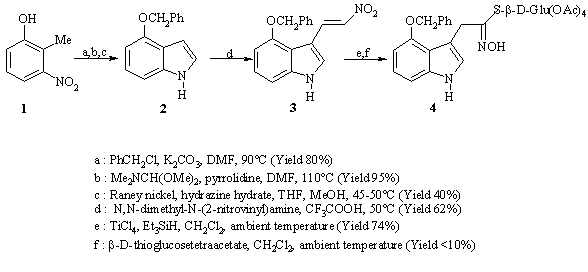
It is well known that 4-hydroxyindole is unstable (Latxague and Gardrat, 1992). In the target compound, 4-hydroxyglucobrassicin, the hydroxyl group needs to be protected. Benzylation has been chosen as a the protection tactic. The synthesis is described in figure 1.
Figure 1 : Preparation of 4-benzyloxydesulfoglucobrassicin
|
Compound 2, 4-benzyloxyindole, was prepared from 2-methyl-3-nitrophenol (1), according to a protocol reported in the literature (Batcho and Leimgruber, 1990). Compound 3, 4-benzyloxy-3-(2-nitrovinyl)indole was then obtained by reacting 2 with N,N-dimethyl-N-(2-nitrovinyl)amine (Severin and Brück, 1965) in the presence of trifluoroacetic acid. The corresponding hydroximoyl chloride was directly prepared from 3 with titanium (IV) chloride and triethylsilane (Kumaran and Kulkarni, 1997 ; Latxague, personal communication). This chloride was then reacted with peracetylated b-D-thioglucose to furnish the peracetylated 4-benzyloxydesulfoglucosinolate (4).
The product 4 has not been yet isolated : the major product of the reaction mixture is formed by the condensation of two sugar molecules. Nevertheless, comparison of 1H NMR spectra of the mixture and of 4-methoxyglucobrassicin (Viaud et al., 1992) is in favour of the presence of the key molecule 4.
CONCLUSION
The preparation of peracetylated 4-benzyloxydesulfoglucobrassicin in six steps from 2-methyl
3-nitrophenol is described for the first time. Nevertheless, yields are to be improved in order to obtain larger quantities of products to synthesize 4-hydroxyglucobrassicin.
EXPERIMENTAL PART
1H and 13C spectra were recorded on a BRUKER AC 250 apparatus (d, ppm). The abbreviations
s (singulet), d (doublet), t (triplet), m (multiplet), br (broad signal) are used.
Only new products are described in this part.
4-benzyloxy-3-(2-nitrovinyl)indole (3) : To a stirred solution of trifluoroacetic acid (1.2 ml) and N,N-dimethyl-N-(2-nitrovinyl)amine (0.3 g) at 0°C was added a solution of 4-benzyloxyindole (0.53 g) in methylene chloride. The mixture was heated to 50°C during 20 min and poured in cold water. After standard work-up, the compound 3 was isolated (0.23 g).
RMN 1H (CD3COCD3) : d 5.22 (s, 2H, CH2Ph) ; 6.85 (m, 1H, H-7) ; 7.08 (m, 1H, H-5) ; 7.19 (m, 1H, H-6) ; 7.35 (m, 3H, CH2Ph) ; 7.64-7.64 (m, 2H, CH2Ph) ; 8.02 (d, 1H, 3JH-8-H-9 = 15.5 Hz, H-9) ; 8.09 (s, 1H, 3JH-2-H-1 = 3 Hz, H-2) ; 8.76 (d, 1H, 3JH-8-H-9 = 15.5 Hz, H-8) ; 11.26 (br, 1H, H-1).
RMN 13 C (CD3COCD3) : d 70.94 (CH2, CH2Ph) ; 104.48 (C-7) ; 106.20 (C-5) ; 109.57 (C-3a) ; 128.58 (C-2) ; 128.81 (2CH, CH2Ph) ; 128.94 (CH, CH2Ph) ; 129.52 (2CH, CH2Ph) ; 131.33 (C-8) ; 134.06 (C-9) ; 135.55 (C-6) ; 138.22 (Cq, CH2Ph) ; 139.22 (C-4) ; 140.19 (C-7a) ; 154.19 (C-3).
4-benzyloxyindol-3-ylhydroximoyl chloride : To a stirred solution of 3 (1.44 g) in methylene chloride were added triethylsilane (0.78 ml) and titanium (IV) chloride (0.82 ml). After two hours at room temperature, the mixture was diluted in water and treated as usual. The product was used without any purification.
4-benzyloxydesulfoglucobrassicin (peracetylated) (4) : To a stirred solution of 4-benzyl-
oxyindol-3-ylhydroximoyl chloride (1.09 g) in methylene chloride (10 ml) and anhydrous ether (20 ml) was slowly added peracetylated b-D-thioglucose under a nitrogen atmosphere. Triethylamine (1.8 ml) in ether (2 ml) was subsequently added. After twelve hours, the mixture was acidified by 1N sulfuric acid (20 ml) and extracted with ethyl acetate. After standard work-up, a mixture of 4 and peracetylated thioglucose dimer was isolated (silica gel)
RMN 1H (CDCl3) (d attributed to peracetylated 4) : d 2.28 ; 2.33 ; 2.38 ; 2.42 (4s, 12H, CH3COO) ; 4.1 (m, 1H, H-5) ; 4.5 (dd, 1H, H-6’’) ; 4.6 (dd, 1H, H-6’) ; 4.7 (d, 2H, H-8’ et H-8’’) ; 5.1-5.4 (m, 4H, H-1, H-2, H-3, H-4) ; 5.2 (s, 2H, CH2Ph) ; 6.7 (dd, 1H, H-7i) ;7.0-7.2 (m, 3H,H-2i,H-5i, H-6i), 7.3-7.5 (m, 5H, CH2Ph) ; 7.8 (br, 1H, H-1i).
REFERENCES
BATCHO, A. D. and LEIMGRUBER, W. 1990. In : Organic synthesis, Coll. Vol. 7, J. P. Freeman Ed., J. Wiley and Sons, New York, pp. 31-34.
FENWICK, G. R., HEANEY, R. K. and MULLIN, W. J. 1983. CRC Critical Reviews in Food Science and Nutrition, 18 : 123-201.
HUANG, M.-T., FERRARO, T. and HO, C.-T. 1994. ACS Symposium Series, 546 : 2-16.
KUMARAN, G. and KULKARNI, H. 1997. Journal of Organic Chemistry, 62 : 1516-20.
LATXAGUE, L. and GARDRAT, C. 1992. Revue Française des Corps Gras, 9-13.
Mc DANNELL, R., Mc LEAN, A. E. M., HANLEY, A. B., HEANEY, R. K. and FENWICK, G. R. 1988. Food and Chemical Toxicology, 26 : 59-70.
NUGON-BAUDON, L. and RABOT, S. 1994. Nutrition Research Reviews, 7 : 205-231.
SEVERIN, T. and BRÜCK, B. 1965. Chemische Berichte, 98 : 3847-53.
SLOMINSKY, B. A. and CAMPBELL, L. D. 1987. Journal of the Science of Food and Agriculture, 40 : 131-143.
TELANG, N. T., KATDARE, H. , BRADLOW, M. L., OSBORNE, M. P. and FISCHMAN, J. 1997. Proceedings of the Society for Experimental Biology and Medicine, 216 : 246-52. (Chemical Abstracts 1998,128 : 18464a).
TOOKEY, H. L., VAN ETTEN, C. H. and DAXENBICHLER, M. E. 1980. In : Toxic Constituents of Plant Foodstuffs, I. E. Liener Ed., Academic Press, New York, 2nd ed. pp. 103-142.
VAN ETTEN, C. H. and TOOKEY, H. L. 1983. In : CRC Handbook of Naturally Occuring Food Toxicants, M. Rechcighl Jr Ed., CRC Press, Boca Raton, pp. 15-30.
VERMOREL, M., DAVICCO, M. J., EVRARD, J. 1987. Reproduction, Nutrition, Développement 27 : 57-66.
VERMOREL, M., HEANEY, R. K. and FENWICK, G. R. 1988. Journal of the Science of Food and Agriculture, 44 : 321-34.
VIAUD, M.-C. and ROLLIN, P. 1990. Tetrahedron Letters, 31 : 1417-1418.
VIAUD, M.-C., ROLLIN, P., LATXAGUE, L. and GARDRAT, C. 1992. Journal of Chemical Research, 1669-81.