TRIACYLGLYCEROLS AND WAX ESTERS IN AUSTRALIAN CANOLA OIL SEDIMENT
Ingrid Bothaa, Kevin Robardsb and Rod Mailera
aNew South Wales Agriculture, Agricultural Research Institute, Wagga Wagga, 2650 ingrid.botha@agric.nsw.gov.au, rod.mailer@agric.nsw.gov.au
bSchool of Science and Technology, Charles Sturt University, PO Box 588, Wagga Wagga, 2650, krobards@csu.edu.au
ABSTRACT
Sedimentation in canola affects the appeal of the oil to consumers. The cause of sedimentation is as yet unknown and there are no definitive tests to detect problem batches of oil before they begin to sediment. Our study was aimed at determining the differences between the oil and sediment, as an indication of the causative components. Palmitic and heptadecanoic acids were higher in sediment of problem oils. Total saturated fats were also higher in the sediment. Sediment from problem oils contained 4 triacylglycerols not found in the clear fraction of the same oils. Each of these triacylglycerols contained palmitic acid. Wax esters were also higher in the sediment than the clear fraction of the same oils. The increased quantity of each of these components suggests that they may contribute to the formation of sediment in canola oil.
KEYWORD canola oil, sediment, wax ester, triacylglycerol, fatty acid
INTRODUCTION
Canola oil usually remains clear without the need for winterisation. However, some canola oils form a sediment during storage, and so is much less attractive to consumers than oil which remains bright and clear. The tendency for oil to form sediment has become an increasing problem in Australia, increasing the requirement for the expensive winterisation process.
Canola oil sediment has been found to consist of triacylglycerols (TAGs), wax esters, free fatty alcohols, free fatty acids and hydrocarbons (for a review see Hermann et al 1999). The major component is either wax esters (Liu et al 1994; Liu et al 1995; Liu et al 1996; Hu et al 1993) or TAGs (Gao & Ackman, 1995). Sediment is formed when crystals develop in the oil and then drop out. These crystals may form around higher melting point components such as long chain wax esters or saturated TAGs.
In this study, we isolated the sediment of problem oils and compared the composition of the sediment fraction to the clear, sediment-free fraction of the same oil. While both fractions contained wax esters, sediment fractions contained more. Total saturated fatty acids were higher in the sediment fraction than the clear fraction. The sediment fraction contained 4 compounds, PPO, PPP, PSO and PPS, which were not detected in the corresponding clear fraction.
MATERIALS AND METHODS
Solvents of chromatographic grade were purchased from Selby-Biolab (Mulgrave North, Australia).
Standards for TAG and fatty acid analysis were purchased from Sigma-Aldrich (Castle Hill, Australia) and included the fatty acid methyl ester reference kit (Oil reference standard, AOCS, O-7756), the olive oil TAG reference kit (TRI-5), and individual TAGs PPP (T5888), PPO (D2282), PPL (D0176) and PLL (D0301). Wax ester standards were purchased from Nu-Check-Prep (Elysian, MN) and included palmityl palmitate, lauryl arachidate, stearyl stearate, behenyl heptadecanoate, arachidyl arachidate, stearyl behenate and behenyl behenate. A range of commercial oil samples were obtained from local supermarkets and manufacturers.
The sediment fraction of problem oils were prepared by centrifugation in a Sorvall centrifuge (20min, 5000rpm, 2oC), then recentrifuged after removal of the supernatant until no further supernatant was evident. The clear fraction was removed from the top of problem bottles and centrifuged once in the same manner, and the supernatant removed for analysis.
Fatty acid profile
Samples were methylated before analysis by GC. Petroleum ether (3mL) was added to 0.1g sample. Sodium methoxide (0.5mL, 2.3g sodium in 200mL anhydrous methanol) was added and vortexed for 15 sec., then left for 10 mins. Indicator (bromothymol blue, 2-3 drops) was added and vortexed. Hydrochloric acid (0.1mL, 1N) then sodium carbonate (0.6mL, 1.5% in water) were added and vortexed. The petroleum ether layer was brought to the top of the test tube by the addition of distilled water, then removed for analysis on a Varian model 3800 gas chromatograph (Mulgrave, Australia) with a BPX-70 fused silica column (30 m x 0.25 mm id, 0.25 mm film) (SGE, Ringwood, Australia). Column temperature was 180oC for 8 min, raised to 220oC at 10oC min-1, and held at 220oC for four minutes. Carrier gas was 1mL min-1 helium. The injector was split/splitless at 250oC, and the detector was a flame ionisation detector at 260oC. Results were integrated using Varian Star Chromatography Workstation software, version 4.5.
Triacylglycerols (TAGs)
Sample (0.1g in 5mL dichloromethane) was analysed on a Waters HPLC system (Waters, Rydalmere, Australia) with a WISP 712 autosampler (15 mL injection) and a Wakosil C18 column (25cm x 4.6mm, 5mm) (SGE, Ringwood, Australia). Elution was isocratic, 32% dichloromethane in acetonitrile over 120 minutes. A Sedex 55 Evaporative Light Scattering Detector (ELSD) (Sedere, Alfortville, France) was used, and the results were integrated using Millennium32 software, version 3.05. Identification of peaks was performed by comparison with standards and also by liquid chromatography-mass spectrometry (LC-MS).
Wax esters
Wax esters were separated from samples using thin layer chromatography (TLC). Sample (0.1g in 100mL chloroform) and internal standard (0.1mg lauryl arachidate in 100mL chloroform) were mixed and applied to silica gel 60 TLC plates (Merck, Kilsyth, Australia) which had been activated by heating at 100oC for 1 hour then cooled. Lauryl arachidate and PPP standards were also applied. Plates were developed with hexane/diethyl ether/acetic acid (80/20/1 v/v/v) (Kattner and Fricke, 1986) and sprayed with 2,7-dichlorofluorescein (0.2% in ethanol).
Wax ester bands were removed from the TLC plate and soaked overnight in chloroform (10mL) and washed twice more with 5mL chloroform. Samples were evaporated to a final volume of 0.5mL, and analysed using a Varian model 3400 gas chromatograph (Mulgrave, Australia) and a BPX-5 fused silica column (10 m x 0.25 mm id; 0.25 mm film) (SGE, Ringwood, Australia). Column temperature was 80oC to 120oC at 30oC min-1, raised to 345oC at 5oC min-1, and held at 345oC for 10 min. Carrier gas was 5mL min-1 helium. Injection was on-column at 320oC and the detector was a flame ionisation detector at 350oC. Results were integrated using Varian Star Chromatography Workstation software, version 4.5.
RESULTS AND DISCUSSION
The TAG profile of clear and sediment fractions of problem oils were compared. Four late eluting TAGs were consistently present in the sediment fraction and were not detected in the clear fraction of problem oils (fig. 1). These TAGs were determined by LC-MS to be PPO, PPP, PSO and PPS. A study by on palm oil (Sulaiman et al 1997) showed that PPP and PPS were two of three saturated triglycerides present in the early stages of sediment formation. PPP has a high melting point of 66oC and so will solidify more quickly than other TAGs. The solidified TAGs may contribute to the nucleation of crystals which then grow and cause the oil to firstly cloud and then form sediment.

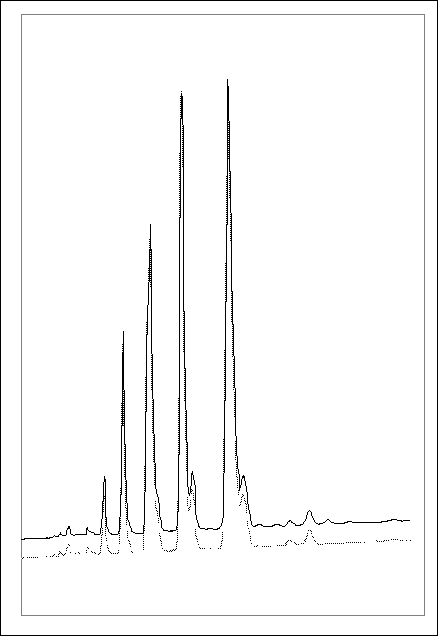
Figure
1: Chromatograms of the clear and
sediment fractions of a problem oil. 4 highly saturated, palmitin containing
TAGs are present in the sediment fraction alone.
Palmitic acid was higher in the sediment fraction than in the clear fraction of the oils studied. Total saturated fatty acids were also higher in all sediment fractions than in the clear oil. These saturated fatty acids have high melting points which may cause them to solidify and act as crystal nuclei.
|
Fatty acid |
Clear fraction |
Sediment fraction |
|
C14:0 |
0.07 |
0.19 |
|
C16:0 |
4.24 |
5.43 |
|
C16:1 |
0.21 |
0.21 |
|
C17:0 |
0.10 |
0.16 |
|
C17:1 |
0.06 |
0.10 |
|
C18:0 |
2.12 |
3.28 |
|
C18:1 |
62.24 |
60.10 |
|
C18:2 |
19.71 |
20.03 |
|
C18:3 |
8.29 |
7.71 |
|
C20:0 |
0.70 |
0.64 |
|
C20:1 |
1.23 |
1.08 |
|
C22:0 |
0.32 |
0.34 |
|
C22:1 |
0.34 |
0.31 |
|
C24:0 |
0.21 |
0.25 |
|
C24:1 |
0.19 |
0.16 |
|
Total saturated fatty acids |
7.76 |
10.28 |
|
Total unsaturated fatty acids |
92.24 |
89.71 |
Table 1: Fatty acid profile of the sediment and clear fractions of problem oils.
Results are the average of 6 oils.
Wax esters were in the range C42 to C52. While wax esters were contained in both fractions, they were present in greater amounts in the sediment fraction. In six samples, the average wax ester content of the clear fraction was 0.34 mg g-1, while the average for the sediment fraction was 1.27mg g-1.
CONCLUSION
The results of our study show that the sediment fraction of problem canola oils contained more total saturated fatty acids than the clear fraction of the same oils. Palmitic acid in particular was higher in the sediment fraction, and four palmitin-containing, highly saturated TAGs were present in the sediment fraction but undetected in the clear oil. Wax esters were also present in greater quantities in the sediment fraction. Each of these components have melting points which may be high enough to allow them to nucleate the crystals which grow and then sediment out. While a number of studies have shown that wax esters are important, TAGs may have an equally important role in sediment formation.
ACKNOWLEDGMENTS
This work was supported by GRDC. The authors would like to thank Daniel Jardine and Julio Hablutzel for their assistance.
REFERENCES
Gao, Z. and R.G. Ackman, J. Sci. Food Agric. 68:421-430 (1995).
Hermann L., R.J. Mailer and K. Robards, Aust. J. Exp. Agric. 39:103-113 (1999).
Hu, X., J.K. Daun and R. Scarth, J. Am. Oil Chem. Soc. 70:535-537 (1993).
Kattner, G. and H.S.G. Fricke, J. Chromatogr. 361:263-268 (1986).
Liu, H., C.G. Biliaderis, R. Przybylski and N.A.M. Eskin, Ibid. 71:409-415 (1994).
Liu, H., C.G. Biliaderis, R. Przybylski and N.A.M. Eskin, Food Chem. 53:35-41 (1995).
Liu, H., R. Przybylski, K. Dawson, N.A.M. Eskin and C.G. Biliaderis, J. Am. Oil Chem. Soc. 73:493-498 (1996).
Sulaiman, M. Z., N.M. Sulaiman and S. Kanagaratnam, Ibid. 74:1553-1558 (1997).