Microspore Culture for Induction of Doubled Haploidy in B. juncea and B. rapa
Dwarkesh S. Parihar, Sanjit Aradhye
Proagro-PGS India Ltd., Dhumuspur Road, Badshahpur, Gurgaon 122001, India
ABSTRACT
Freshly isolated microspores from B. juncea (Indian Mustard) were cultured in NLN medium at a density of 3.5x104/ml in dark at 30oC formed embryos in 10-15 days time. Addition of activated charcoal resulted in 0.1% of the cultured microspores forming embryos. Higher or lower culture density of the microspores adversely affected development of embryos. Strong genotypic effect was also observed in microspore embryogenesis. Microspore embryos were allowed to grow and regenerate on B5 medium supplemented with 0.01 mg/l GA3. Chromosome doubling was carried out with Colchicine and ca. 60% of the plants transferred to green house were fertile and set selfed seeds. Microspore embryogenesis was also possible in B. rapa using almost similar methodology with certain modifications. The microspores cultured at a density of 2.0x104/ml in dark at 25oC formed embryos in 10-15 days time. The microspore embryogenesis response was comparable to B. juncea and genotypic differences were evident.
KEY WORDS: B. juncea, B. rapa, microspore embryogenesis, doubled haploidy
Introduction
Doubled haploidy is a unique tool to derive additional variability in the germplasm and achieve homozygosity. In vitro androgenesis and gynogenesis are usually the method of choice for regeneration of haploids. Normally androgenesis by microspore culture is successful in B. napus (Keller et al. 1975, Thomas and Wenzel 1975, Lichter 1982). Haploidy has been reported in B. juncea by anther as well as microspore culture (George and Rao 1982, Sharma and Bhojwani, 1985, Prabhudesai and Bhaskaran 1993, Thiagarajah and Stringam 1993) and in B. rapa (Burnett et al., 1992, Guo and Pulli 1996).
Further, high efficiency microspore culture has a potential to increase efficiency of the breeding program through use of doubled haploid lines. Such a breeding strategy is particularly attractive as it not only provides an opportunity to select at the haploid level in vitro for desirable agronomic traits, but also provides a rapid means of producing genetically stable homozygous lines, fixed by chromosome doubling. Present study outlines our efforts to induce microspore embryogenesis in B. juncea and B. rapa, important oilseed crops for India.
Materials and Methods
Two cultivated varieties if B. juncea and B. rapa were selected to develop and efficient microspore culture procedure for oilseed breeding program of Proagro-PGS India Ltd. The donor plants were grown in green house under natural conditions. The flower buds were harvested in the morning and sterilized by 4% Sodium hypochlorite for 10 min. The buds were homogenized in B5 liquid medium containing 13% sucrose, in a waring blender and the homogenate was centrifuged 3 times at 1100 rpm for 3 min to harvest pure microspore preparation. The purified microspores were resuspended in medium proposed by Lichter (1982), lacking hormones. The microspores were incubated at 30oC in dark for 15 days. Once the embryos were visible by naked eye the culture plates were transferred to a shaker at 60 rpm in dark at 25oC for 7-10 days.
The cotyledonary stage embryos were transferred to B5 medium fortified with 0.01 mg/l GA3 and solidified with 0.6% agar. The culture were provided with 16h/8h light cycle at 25oC. The plantlets were transferred to same medium in plastic boxes for further development. The fully developed plants were treated with colchicine and transferred to green house for flowering and harvesting of selfed seeds.
Results and Discussion
The microspore cultures from B. juncea and B. rapa responded to culture conditions and formed microspore embryos medium proposed by Lichter (1982). The embryo yields were significantly affected by the developmental stage of microspore. The optimum response was obtained from mid-late uninucleate stage. Further it was possible to establish useful correlation between developmental stage of microspores and bud size (Fig. 1). Similar response was observed by Kott et al. (1988) in B. napus and by Gou and Pulli (1996) in B. rapa. Guo and Pulli (1996) also mentioned that the correlation between optimum bud size and microspore developmental stage varies with the genotype.
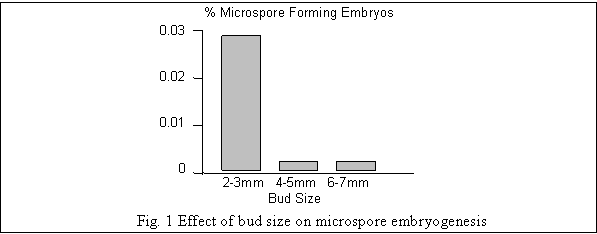
The density of microspore suspension during culture initiation affected the initiation and development of embryogenesis. Highest yield of microspore embryos was obtained from a culture density of 1x104/ml. Although, initiation of embryogenesis was observed at higher as well as lower density, but the development into later stages was adversely affected, especially in high density cultures (Fig. 2). Further, delay/arrest of development of microspore embryos in high density cultures can also be attributed to limited availability of nutrients or accumulation of inhibitory compounds in prolonged cultures.
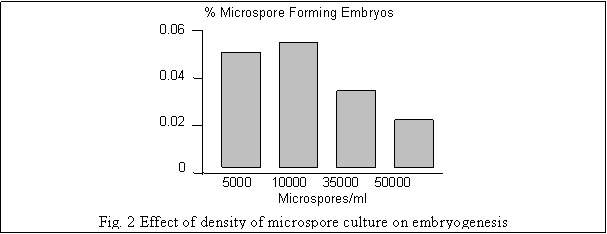
Johansson (1983) proposed addition of activated charcoal to the medium for improving microspore embryogenesis in some plants. In the present study addition of activated charcoal in the microspore cultures contributed positively towards development of microspore embryos (Fig. 3). However, it appears that charcoal does not increase the number of embryos but it promotes the development of embryos so that they regenerate more easily and develop into whole plants. Possibly activated charcoal acts by adsorbing inhibitors from the growth medium in prolonged culture period (Gland et al. 1988).
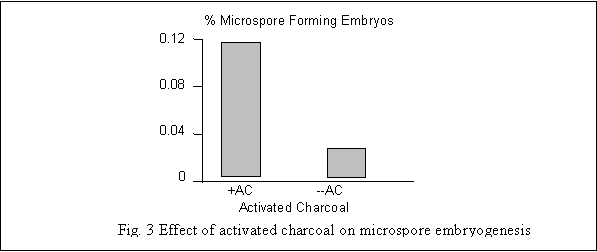
Among other factors, concentration of sucrose was found to affect microspore embryogenesis, but the differences were not very significant. However, 13% sucrose gave optimum response (data not presented).
Using similar approach it was possible to obtain microspore embryogenesis in B. rapa. Certain modifications, like incubation of microspores at 25oC in dark and a culture density of 2x104 /ml were found to be optimum. It was possible to obtain complete plants from 60-70% microspore embryos. Also, chromosome doubling by Colchicine resulted in ca. 60-70% fertile plants forming good selfed seed set.
CONCLUSION
The results of the present study indicate possibility of obtaining DH lines in Indian germpalsm of B. juncea and B. rapa. The microspore embryogenesis response is primarily affected by developmental stage of microspores, density of cultures during incubation and presence of activated charcoal. The DH lines obtained by the method developed are being extensively used in oilseed breeding program of Proagro-PGS India Ltd.
ACKNOWLEDGEMENTS
We wish to thank Dr A. Reynaerts, F. Leyns, K. Lievens of Plant Genetic Sytems, Gent, Belgium, for help and advise.
REFERENCES
Burnett, L., Yarrow, S., Huang, B. 1992. Plant Cell Rep. 11:215-218.
George, L. and Rao, P.S. 1982. Plant Sci, Lett. 26:111-116
Gland, A., Lichter, R., Schweiger, H.-G. 1988. J. Plant Physiol. 132:613-617.
Guo, Y. And Pulli, S. 1996. Plant Cell, Tissue and Organ Culture 46:219-225.
Johansson, L. 1988. Physiol. Plant. 59:397-403.
Keller, W.A., Rajhaty, T., Lacapra, J. 1975.. Can. J. Genet. Cytol. 17:655-666.
Kott, L.S., Polsoni, L., Ellis, B., Beversdorf, W.D. 1988. Can. J. Bot.
Lichter, R. 1982. Z. Pflanzenphysiol. 105:427-434.
Prabhudesai, V. and Bhaskaran, S. 1993. Plant Cell Rep. 12:289-292.
Sharma, K.K. and Bhojwani, S.S. 1985. Plant Cell Tissue and Organ Culture. 4:235-239.
Thiagarajah, M. and Stringam, G.R. 1993. Plant Breeding. 111:330-334.
Thomas, E. and Wenzel, G. 1975. Z. Pflanzenzuchtg. 74:77-81.