NEW ALLOPLASMATIC FORMS OF WINTER OILSEED RAPE CREATED BY INTERSPECIFIC CROSSING
M. Starzycki, E. Starzycka, M. Matuszczak, J. Krzymanski
Plant Breeding and Acclimatization Institute, Poland, 60-479 Poznan, Strzeszynska 36
e-mail: michals@nico.ihar.poznan.pl
ABSTRACT
Studies on the interspecific hybrids within Brassicaceae K. family were done to increase the diversity of economically important traits of oilseed rape. The target of these works was to obtain new yellow seeded genotypes or genotypes having Brassica oleracea L. cytoplasm. Embryo rescue method connected with in vitro culture was used to produce hybrids between B. oleracea var. gemmifera (D.C.), B. oleracea var. acephala, B. oleracea var. acephala subvar. Lacinista and B. napus. These hybrids were backrossed 5 times using B. napus lines as pollinators. Obtained new lines were morphologically similar to recurrent parent and difficult to distinguish from B. napus pollinator lines. Search for molecular markers characteristic to cytoplasm of used three B. oleracea form are conducted now.
KEYWORD: Brassicaceae, in vitro culture, interspecific hybrids, molecular markers
INTRODUCTION
Many scientists all over the world started the studies on interspecific hybrids within Brassicaceae K. family (Batra et al. 1990; Brown et al. 1997; Earle et al. 1992; Gundimeda et al. 1992; Landgren and Glimelius 1994; Liu et al. 1995; Nanda Kumar and Shivanna 1993; Nothnagell et al. 1997; Sundberg and Glimelius 1991; Wojciechowski 1985; Wojciechowski et al. 1997). Most of them tried to construct new alloplasmatic forms that carry traits of both parental species. Such studies were also conducted in Plant Breeding and Acclimatization Institute in Poznan to obtain new yellow seeded genotypes and genotypes having Brassica oleracea L. cytoplasm. The main goal of these works is to increase cold-resistance and resistance to some fungal pathogens of Brassicaceae K. An important step is the transfer of cytoplasm to Brassica napus L. lines of high quality after the initial interspecific crossing. This was done using several backcrossing with Brassica napus L. lines as pollinators. After four backcrosses of hybrids with Brassica napus L. the offspring was morphologically similar to the pollinator line. Because of the problems with visual identification of hybrid BC4 plants some authors suggest the use of mtDNA, ptDNA or isoenzymatic markers as a tool for such identification (Nothnagel et al. 1997; Brown et al. 1997, Earle 1992, Landgren and Glimelius 1994).
MATERIALS AND METHODS
The following varieties of Brassica oleracea L. were used as the source of cytoplasm: Brassica oleracea var. gemmifera (2n=18), Brassica oleracea var. acephala (2n=18) and Brassica oleracea var. acephala subvar. Lacinista (2n=18). Winter oilseed rape (Brassica napus L.) (2n=38) was used as pollinator. Parents at the stage of eigh leaves were vernalized for ten weeks and then were transferred to the greenhouse. During the flowering phase the interspecific crossing was performed - to obtain the progeny with Brassica oleracea L. cytoplasm. The hybrid embryos were isolated three weeks after pollination. Globular forms were transferred on B5 medium with 10% sucrose. Larger embryos were also put on B5 medium but the concentration of sucrose was only 3%. All plants were rooted in hydroponic culture and then moved to the pot culture - to produce the hybrids with the highest possible efficiency. Starting with HF1 generation the plants were backcrossed with winter oilseed rape as pollinator according to the scheme below:
B. oleracea x B. napus year I
(n = 9) ¯ (n = 19)
HF1 x B. napus year II
(hybrid 28) ¯
BC1 x B. napus year III
¯
BC2 x B. napus year IV
¯
BC3 x B. napus year V
¯
BC4 x B. napus year VI
Number of chromosomes was determined for parents as well as for hybrid HF1 and BC4 plants using orcein method. Isolation of total DNA was made as described by Pastuglia et al. (1997). PCR analyses were performed using pair of primers for coxIII gene and two RAPD primers (Operon). The PCR reaction mixture for detection of coxIII gene contained: 100mM of each of dATP, dGTP, dCTP and dTTP (MBI Fermentas), 5pmoles of each coxIII gene primer, 0.75units of Taq polymerase with buffer (Gibco BRL), 1.5mM MgCl2 and 50ng of genomic DNA. The final reaction volume of 25ml was obtained by adding of double distilled water. Amplification was conducted with „hot start” in the Biometra UNOII thermocycler with the following reaction parameters: initial template denaturation (without Taq polymerase) - 98°C, 7min.; amplification - 30 cycles of denaturation: 94°C, 1min., annealing: 55°C, 1min. and polymerization: 72°C, 2min.; final polymerization - 72°C, 10min. The PCR-RAPD reaction mixture contained: 150mM of each of dATP, dGTP, dCTP and dTTP (MBI Fermentas), 0.2mM 10bp-long RAPD primer (Operon), 0.4units of Taq polymerase with buffer (Eurobio), 1.9mM MgCl2 and 12.5ng of genomic DNA. The final reaction volume of 12.5ml was obtained by adding of double distilled water. Amplification was conducted in the Biometra UNOII thermocycler with the following reaction parameters: initial template denaturation - 94°C, 30sec.; amplification - 45 cycles of denaturation: 94°C, 30sec., annealing: 35°C, 1min. and polymerization: 72°C, 2min.30sec.; final polymerization - 72°C, 5min. All reaction products were separated using agarose gel electrophoresis. Agarose concentration was 1.4% for coxIII gene-specific PCR products and 1.8% for RAPD products. Separated fragments were visualized under ultraviolet light after ethidium bromide staining. The size marker used was Lambda DNA digested by HindIII and EcoRI (MBI Fermentas).
RESULTS
For every combination of parents the sterile F1 generation could be considered as hybrid because this progeny carried phenotypic characters of both ancestors.
Table 1: Comparison of the number of in vitro embryos and hybrid plants obtained
|
Type of crossing |
Number of isolated „ovules” |
Number of obtained embryos |
Number of obtained plants |
|
B. oleracea var. gemmifera x B. napus („00”)
|
1500 |
12 |
65 |
|
B. oleracea var. acephala x B. napus („00”) subvar. Lacinista
|
700 |
53 |
20 |
|
B. oleracea var. acephala x B. napus („00”)
|
621 |
15 |
53 |
|
å |
2821 |
80 |
138 |
All of the HF1 plants had 28 chromosomes in somatic cells (ACC=28) and BC4 plants had 38 chromosomes. After six years of crossing new forms of double-zero rapeseed were obtained. PCR analyses were performed to confirm the presence of cabbage cytoplasm in these plants.
|
|
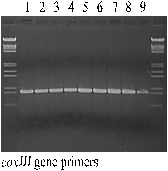
Figure 1: PCR analysis for detection of mitochondrial coxIII gene; 1 - parent B.oleracea var. gemmifera; 2 - parent B.oleracea var. acephala subvar. Lacinista; 3 - parent B.oleracea var. acephala; 4 - pollinator B.napus L.; 5 - HF1 of the cross B.oleracea var. acephala ´ B.napus L.; 6 - HF1 of the cross B.oleracea var. gemmifera ´ B.napus L.; 7 - BC4 of the cross B.oleracea var. gemmifera ´ B.napus L.; 8 - BC4 of the cross B.oleracea var. acephala subvar. Lacinista ´ B.napus L.; 9 - BC4 of the cross B.oleracea var. acephala ´ B.napus L. |
The pair of coxIII mitochondrial gene specific primers caused the amplification of expected fragment in all cases.
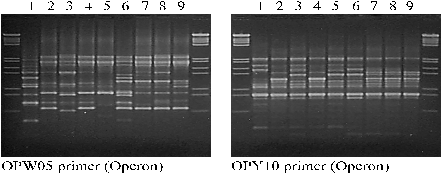
Figure 2: PCR-RAPD analyses with OPW05 and OPY10 primers; 1 - parent B.oleracea var. gemmifera; 2 - parent B.oleracea var. acephala subvar. Lacinista; 3 - parent B.oleracea var. acephala; 4 - pollinator B.napus L.; 5 - HF1 of the cross B.oleracea var. acephala ´ B.napus L.; 6 - HF1 of the cross B.oleracea var. gemmifera ´ B.napus L.; 7 - BC4 of the cross B.oleracea var. gemmifera ´ B.napus L.; 8 - BC4 of the cross B.oleracea var. acephala subvar. Lacinista ´ B.napus L.; 9 - BC4 of the cross B.oleracea var. acephala ´ B.napus L.
The pattern of RAPD-generated bands for all BC4 plants having cabbage-originated cytoplasm is identical. The largest differences can be observed for F1 generation of hybrids. RAPD primers were very useful for identification of three different cabbage varieties utilized in our experiments. The bands pattern of Brassica oleracea var. acephala subvar. Lacinista seems to be very similar with that of winter oilseed rape (Brassica napus L.).
DISCUSSION
Most of the world reports about interspecific hybrids within Brassicaceae K. family are focused on crossing between taxonomically distant species like Sinapis alba L. ´ Brassica napus L. and Sinapis alba L. ´ Brassica oleracea L. (Nothnagel et al. 1997; Brown et al. 1997). For the former crossing the in vitro culture of isolated embryos and for the latter the protoplast fusion was used. In our studies on Brassica oleracea L. and Brassica napus L. we used only the in vitro culture of isolated embryos (Tab. 1). There are lots of difficulties when trying to cross different species. The main problem is that both germination of foreign pollen grains on the stigma surface and penetration of pollen tube into the style are usually stopped for such crosses. Members of Brassicaceae K. family have sporophytic incompatibility system coming from mother plant and causing problems mentioned above (Nettancourt 1969). In many cases after overcoming of sporophytic incompatibility further endosperm and embryo incompatibilities must be eliminated through in vitro cultures. After hybrid pollination - if there is no siliques formation - we can consider that sporophytic incompatibility occurred and if embryos are deformed there is possible endosperm incompatibility. Hybrid crossing results in breaking of the harmony between endosperm, embryo, seed coat and pericarp - first symptoms can be observed in endosperm as non-typical mitoses and tumorous growth. This abnormal endosperm development can result in weakness and subsequent necrosis of embryo. However, if isolated on the early stage, such embryos can be maintained in vitro on the medium and normal growth can be achieved. During our studies we did not observe any growth perturbations if embryos are put on the medium in heart or torpedo stage. For globular embryos moving them on the medium usually caused necrosis. Cytological studies of HF1 plants proved that they are hybrids because all of them had 28 chromosomes (ACC=28). Another evidence for that is sterility caused by the lack of pollen grains as well as leaves morphology and the general look of descendant plants - very similar to the mother forms. The BC4 plants are looking like winter oilseed rape and are difficult to distinguish from Brassica napus L. pollinator lines. To identify them we tried to find cytoplasmic molecular markers. Marker for coxIII gene was present in all studied samples - the only conclusion from that result is that we had proper method for isolation of cytoplasmic DNA (Fig. 1). This marker did not differentiate analyzed plants according to cabbage- and rapeseed-derived cytoplasm, so we had to use other primers to identify them. Patterns of RAPD bands also were not good enough to distinguish new forms of rapeseed having cabbage cytoplasm from their ancestors (Fig. 2). We suggest further investigations to find better cytoplasmic markers.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge dr hab. J. Sadowski, dr D. Babula and dr M. Jedryczka - IGR PAN Poznan and prof. dr hab. H. Augustyniak - UAM Poznan for help in DNA isolation.
LITERATURE
Batra V., Prakash S., Shivanna K., R. 1990. Intergeneric hybridization between Diplotaxis siifolia, a wild species and crop brassicas. Theoretical and Applied Genetics, 80: 537 - 541.
Brown J., Brown A. P., Davis J. B., Erickson D. 1997. Intergeneric hybridization between Sinapis alba and Brassica napus. Euphytica 93: 163- 168.
Earle E. D., Temple M., Walters T.W. 1992. Organelle assortment and mitochondrial DNA rearrangements in Brassica somatic hybrids and cybrids. Physiologia Plantarum, 85: 325 - 333.
Gundimeda H. R., Prakash S., Shivanna K. R. 1992. Intergeneric hybridization between Enarthrocarpus lyratus, a wild species and crop brassicas. Theoretical and Applied Genetics, 83: 655 - 662.
Landgren M., Glimelius K. 1994. A high frequency of intergenomic mitochondrial recombination and an overall biased segregation of B. campestris or recombined B. campestris mitochondria were found in somatic hybrids made within Brassicaceae. Theoretical and Applied Genetics, 87: 854 ‑ 862.
Liu J. H., Dixelius C., Eriksson I., Glimelius K. 1995. Brassica napus (+) B. tournefortii, a somatic hybrid containing traits of agronomic importance for rapeseed breeding. Plant Science, 109: 75 ‑ 86.
Nanda Kumar P. B. A., Shivanna K. R. 1993. Intergeneric hybridization between Diplotaxis siettiana and crop brassicas for the production of alloplasmic lines. Theoretical and Applied Genetics, 85: 770 -776.
Nothnagel T., Budahn H., Straka P., Schrader O. 1997. Successful backcrosses of somatic hybrids between Sinapis alba and Brassica oleracea with the Brassica oleracea parent. Plant Breeding, 116: 89 - 97.
Nettancourt, 1969. Badation effects on one Locus-gametophytic system on Self-incompatibility in higher plants. Theoretical and Applied Genetics, 39: 187 -196.
Putnam L. G., 1977. Response of four Brassica seed crop species to attack by the crucifer flee beetle, Phyllotreta cruciferae. Canadian Journal of Plant Science, 57: 987 - 989.
Pastuglia M., Ruffio-Chable V., Delorme V., Gaude T., Dumas C. Cock M. 1997. A functional s locus anther gene is not required for the self - incompatibility response in Brassica oleracea. The Plant Cell. Vol. 9 2065 - 2076.
Sundberg E., Glimelius K. 1991. Production of cybrid plants within Brassicaceae by fusing protoplasts and plasmolytically induced cytoplasts. Plant Science 79: 205 -216.
Wojciechowski A. 1985. Interspecific hybrids between Brassica campestris L. and B. oleracea L. I. Effectiveness of Crossing , Pollen Tube Growth, Embriogenesis. Genetica Polonica, 26/4: 423 - 436.
Wojciechowski A., Nowak K., Olejniczak J. 1997. Wstepne wyniki krzyzowan miedzygatunkowych pomiedzy Brassica napus, B. oleracea, B. campestris i B. fruticulosa. Rosliny Oleiste, tom XVIII: 91 - 98.