AN IN VITRO MUTAGENESIS – SELECTION SYSTEM FOR
BRASSICA NAPUS L.
Teresa Cegielska-Taras, Laurencja Szała, Jan Krzymański
Plant Breeding and Acclimatization Institute – Strzeszyńska 36, 60-479 Poznań, Poland
ABSTRACT
Two types of mutagenes were used to generate genetic variation in microspore culture of oilseed rape: ultraviolet (UV) radiation and chemical mutagen N-methyl-N-nitroso urea (MNU). Embryos derived from mutated microspores were placed in liquid medium B5 with addition of the selective agent phosphinothricin (PPT), an active ingredient of herbicide Liberty (AgrEvo). Within a few days only surviving embryos were transferred to agar plates for further development. The next resistance test was conducted on M1 progeny of DH mutant lines.
KEYWORDS: microspore culture, mutation, MNU, UV light, herbicide
INTRODUCTION
Doubled haploids (DH) have recently become a way to significant shortening of the breeding cycle of oilseed rape (Brassica napus L.). This system is fully compatible with other biotechnological approaches such as mutation or gene manipulation techniques (Szarejko et.al.,1991). Mutagenic treatment of a single, potentialy totipotent haploid cell, in this case of isolated microspore, followed by rapid differentiation in the embryo, represents a unique opportunity for screening homozygotic, non-chimerical M1 organisms for resistance against particular factor.
The main goal of this study was to get doubled haploid lines resistant to PPT, active ingredient of herbicide Liberty (AgrEvo), from embryos after in vitro selection for resistance to PPT derived from isolated microspores after UV or MNU treatment .
MATERIALS AND METHODS
Donor plants of microspores were homozygous line, DH-O120, of double low winter oilseed rape. Growth conditions of these plants and microspore isolation method were as described previously (Cegielska-Taras and Szała, 1997).
Mutagenic treatment of microspores
UV- radiation. The isolated microspores were subjected to UV radiation immediately following the fourth centrifugation of the isolation procedure. The pellet was resuspended in the NLN-medium (Lichter, 1982) with 13 % of sucrose (NLN-13) and 12 ml of resuspended microspores were transferred to 90 mm Petri dishes. The open dishes were placed in a laminar flow bench at a distance of 250 mm from a UV tubes (Philips-TUV 30 W, major emission at 254 nm) for various time intervals. Immediately after the radiation dishes were wrapped in aluminium foil and placed at 30°C in the dark. The medium was changed using centrifugation in the next day of culture. The pellet was resuspended in the NLN-13 medium to concentration of 40 000 microspores/ml.
MNU-treatment. The isolated microspores were treated with MNU immediately following the fourth centrifugation of the isolation procedure. The pellet was resuspended in the NLN-13 medium with 100 mM MNU. After 22 h of culture at 30° C the medium was changed by centrifugation. The pellet was washed twice with fresh culture medium and finally resuspended in the NLN-13 medium to concentration of 40 000 microspores/ml.
Embryos culture. Treated with UV or MNU microspores were incubated at 30°C for 8-10 days and then transferred to a slow rotary shaker in the light at 25° C. Small cotyledonary microspore-derived embryos (MDEs) were passed onto the NLN medium with 8% of sucrose (NLN-8) for two days.
In vitro selection for resistance to PPT
Young embryos were transferred onto liquid B5 medium (Gamborg et al. 1968) with 2% sucrose and supplemented with 40 mg/l PPT ( active ingredient of herbicide Liberty). Petri dishes were placed on a slow rotary shaker in the light at 24° C. After two weeks the embryos which remained green and were developing normally in the presence of the PPT were transferred on the solid NLN medium with 2 % of sucrose (NLN-2). These embryos were subcultured to regeneration medium which stimulates plantlets formation, as described previously (Cegielska-Taras and Szała, 1997). A number of plantlets have been regenerated, colchicine doubled and collected selfed seeds.
Testing obtained DH lines for resistance to herbicide
Seeds (M1 generation ) collected from 22 DH lines obtained from survived MDEs were sown. The five plants per each DH line were used to test for resistance to herbicide. At the stage of four leaves seedlings were sprayed with herbicide Liberty (AgrEvo) with a dose 4l/ha.
RESULTS AND DISCUSSION
The microspore mutagenesis and selection system can produce a plant population with geneticaly fixed novel traits. The advantage of a large-scale microspore selection system over traditional somatic tissue selection systems are evident (Polsoni et al., 1987). This technique involves applying a mutagenic treatment, physical (UV), or chemical (MNU), to embryogenic microspores. Embryos developing from mutated microspores can be regenerated into plants. Selection at the embryo stage is the rapid way especially in the case of herbicide tolerant mutants. The chemical factor can be incorporated directly into the culture medium and potential mutants can be selected very easily. Several canola cultivars have resistant to herbicides: imidazolines (Swanson et al. 1989), chlorsulfuron (Ahmad et al.1991) and glyphosate (Kott, 1996) have been developed using in vitro mutagenesis and selection.
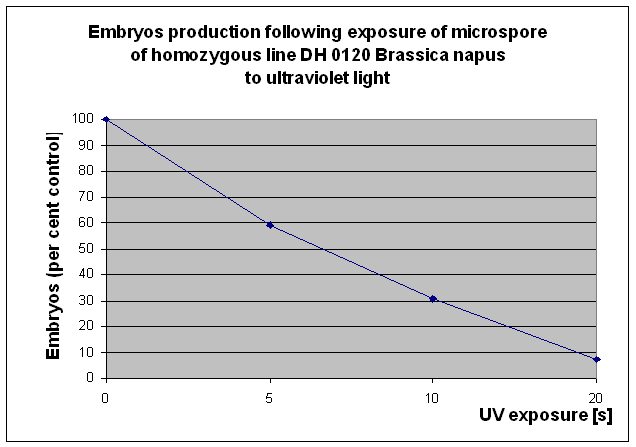
This system has been investigated on microspores treated with mutagens: UV or MNU, immediately after isolation. Reduction of embryogenesis at the 50% rate, (LD50) for microspores after UV treatment is achieved after 7 sec. (Fig. 1.). But to obtain higher mutation ratio in this experiment the microspores were exposed to UV for 10 sec. In the case of MNU several doses: 50, 75, 100, 150, 200 mM. were examined. The dose 100 mM gave just a little more than 50% reduction of microspore embriogenesis. Young embryos had been found to be inhibited by the used selective agent, in dose 40 mg/l PPT. Plants were regenerated from the survived MDEs which were subcultured once or twice until normal leaves and roots were formed. Up till now, 42 androgenic plants were regenerated from embryos which survived medium with PPT developed from microspores after UV radiation, and 7 androgenic plants from embryos surviving selection medium developed from microspores which were treated with MNU. Among regenerated plants spontaneous doubling of chromosomes was observed very often. The seeds obtained from 22 DH lines were used in the experiment. These DH lines were derived from embryos which survived selective medium and developed from microspores after having been exposed to UV light. The colchicine treatment was carried out in three androgenic plants only. At the stage of four leaves five plants out of each 22 DH lines were sprayed with herbicide Liberty. Plants of homozygous line DH-O120 which was the donor of microspores were used as control. The results of the test indicated, that only one DH line was a little more tolerant to the studied herbicide than the other tested and than the control plants. The subsequent test of Liberty tolerant of the selected DH line will be carried on.
The results of the study indicate that embryos at in vitro stage and the progeny of plants developed from survived embryos demonstrate different level of resistance to herbicide. From 22 tested DH lines obtained from embryos survived on selective medium one line was a little more tolerant to herbicide Liberty (AgrEvo) only.
The system in vitro mutagenesis-selection on microspore-embryos level is very convenient for generating and discovering a novel genetic variation in Brassica napus L. especially when microspores from homozygous donor plants were used. Then it can supposed, that every novel trait of DH lines regenerated from treated microspores will be mutagenic change.
REFERENCES:
Ahmad I., Day J.P., MacDonald M.V., Ingram D.S. 1991. Haploid culture and UV mutagenesis in rapid-cycling Brassica napus for the generation of resistance to chlorsulfuron and Alternaria brassicicola. Annales of Botany 67, 521-525.
Cegielska-Taras T., Szała L. 1997. Plants regeneration from microspore-derived embryos of Brassica napus L. Rośliny Oleiste – Oilseed Crops, XVIII, 21-30.
Gamborg O.L., Miller R.A., Oijma L. 1968. Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research 50, 151-158.
Kott L. 1996. Doubled haploid technology accelerates canola breeding. Agri-Food Research in Ontario. 19, 16-18.
Lichter R. 1982. Induction of haploid plants from isolated pollen of Brassica napus L. Z.. Pflanzenphysiol. 105, 427-434.
Polsoni L., Kott L.S, Beversdorf W.D. 1988. Large-scale microspore culture technique for mutation-selection studies in Brassica napus. Can. J. Bot. 66, 1681-1685.
Swanson E.S., Herrgesell M.J., Arnoldo M., Sippell D., Wong R.S.C. 1989. Microspore mutagenesis and selection: canola plants with field tolerance to the imidazolinones. Theoretical and Applied Genetics. 78, 525-530.
Szarejko I., Małuszyński M., Polok K., Kilian A. 1991. Doubled haploid in the mutation breeding of selected crops. Proceedings of an International Symposium on the Contribution of Plant Mutation Breeding to Crop Improvement. 2, 355-378.